This article was originally written and published in Microbiologist in 2016. Therefore, certain wording may reflect this.
It is now clear that many important metal resources are threatened by over-exploitation, inadequate recycling and reclamation, and geopolitical issues. Consumption of metals and minerals has increased steadily in recent decades and rising population growth and prosperity ensures that demand will accelerate. The rise of mega-cities has also exacerbated problems of metal recycling and reclamation and has given rise to the concept of “urban mining” which seeks to improve recovery of important elements from urban waste. Furthermore, the increasing need for energy-efficient electronic materials such as those used in computers, mobile phones and televisions is highly dependent on a specific range of metal and mineral resources. Such “E-tech elements” include cobalt, platinum group metals and rare earth elements, as well as metalloids like selenium and tellurium. Some of these elements are already in short supply and can be difficult to recover by conventional mining and extraction. Being found in only a small number of geographic locations, this also renders the supply chain extremely vulnerable to economic and political forces. The EU is almost wholly dependent on imported supplies and it is, therefore, generally accepted that there is an urgent need to improve the global security of supply of important elements. The EU is also eager to balance new mining processes with the requirements of minimizing environmental impact such as pollution and increased greenhouse gas emissions. Of the many worldwide initiatives designed to address this problem, microbial bioprocessing is seen as an essential component of the approaches that may be used to improve metal recovery.
The ability of microorganisms to change the chemical speciation of metals is well known and is of profound significance in the natural biogeochemical cycles for metals and associated elements in rocks, minerals, soil and organic matter. A variety of “bioweathering” mechanisms are involved in the microbial attack of rocks and minerals, including physical penetration and the production of acidic and/or metal complexing metabolites such as organic and inorganic acids, and siderophores. Bioreduction can also result in increased solubility, e.g. Mn(IV) to Mn(II). Such solubilisation mechanisms provide a means of metal biorecovery from solid matrices. The application of bacterial bioleaching using, e.g. Acidithiobacillus spp., is a well-established industrial process for several metals, for example copper from mineral ore resources, and such mechanisms also may be applicable to recovery of elements from other industrial and electronic wastes. Conversely, metals and metalloids can be immobilised as elemental or biomineral forms, e.g. Ag0, Se0, Te0 and manganese oxides through redox transformations as well as by the production of metabolites such as sulphide, oxalate and CO2 that lead to metal precipitation as sulphides, oxalates and carbonates respectively. Biomineralisation can also be mediated through release of anionic substances that combine with metals (e.g. phosphates, carbonates and sulphates) as a consequence of mineral dissolution or from the biodegradation of organic substrates. The variety of biomineralisation mechanisms used by microorganisms provides a means of recovering elements from leachates, process streams and effluents.
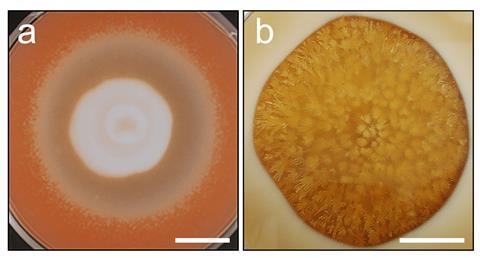
The growing discipline of geomicrobiology, which seeks to understand roles of microorganisms in geological processes, encompasses many aspects of microbial metal and mineral biotransformations. Species from all microbial groups can effect geochemical transformations, with the great metabolic diversity of prokaryotes ensuring that these organisms receive considerable research attention, especially regarding redox transformations in the subsurface. Fungi are less appreciated as geoactive agents but are of profound importance in the terrestrial environment and capable of many metal and mineral transformations. Many geomycological processes are relevant to natural cycling of elements, rock and mineral transformations, plant productivity and biodeterioration. There is also growing appreciation of their geoactive potential in the context of metal and mineral transformations and biorecovery.
Fungi are ideally suited as geoactive agents, and the vast majority exhibit a branching filamentous explorative lifestyle. They are chemoorganotrophic and excrete a variety of extracellular enzymes and metabolites that interact with inorganic and inorganic substrates. Their geoactive properties are underpinned by their metabolism and lifestyle and they can directly and indirectly mediate the formation of many kinds of minerals, including oxides, phosphates, carbonates and oxalates, as well as elemental forms of metals and metalloids such as Ag, Se and Te. Such biomineralisation largely depends on the organism modifying its local microenvironment to create appropriate physico-chemical conditions for precipitation to take place and this can depend directly and indirectly on metabolism. Compared to the simpler bacterial cell form, the filamentous fungal growth habit may additionally provide more framework support and stability as a reactive network for biomineralisation.
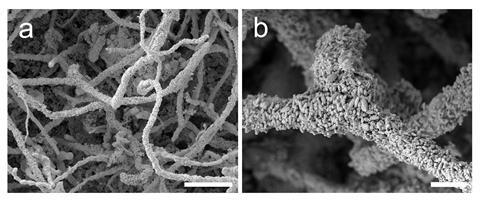
Fungi are very important biodegraders of organic material and can indirectly result in mineral formation where certain biodegradation products react with available metal species. For example, the action of phosphatase enzymes on P-containing organic substrates results in the release of inorganic phosphate that can then precipitate with available metals. Such fungal phosphatase-mediated metal bioprecipitation can extensively precipitate lead or uranium phosphates on hyphal surfaces during growth on a source of organic phosphorus in the presence of soluble Pb and U and removal from solution of potentially toxic or radioactive elements is of great bioremedial potential. A previous discovery demonstrated the fungal-mediated formation of pyromorphite, a lead phosphate, from metallic lead. Pyromorphite is extremely insoluble and can form in Pb-polluted soil especially when a source of inorganic phosphate is applied in abiotic soil remediation treatments. It is highly likely that soil fungi are mediating pyromorphite formation in such locations resulting in lead immobilisation. Similarly, the formation of stable uranium phosphate minerals during fungal growth in the presence of uranium oxides or depleted uranium has also been demonstrated.

Microbially-mediated carbonate precipitation has been used for metal and radionuclide bioremediation, soil stabilisation and the reinforcement of concrete structures. Such a system may also provide a promising method for the biorecovery of toxic or valuable metals (e.g. Co, Ni, and La). Many free-living fungi are capable of urea degradation resulting in alkalinisation of their environment due to the production of carbonate that precipitates with any susceptible metals present. Urease-positive Neurospora crassa can precipitate calcite (CaCO3), as well as other metal carbonates, when incubated in urea-amended media. Biomass-free culture supernatants also have precipitative properties which facilitates metal biorecovery from solution in pure form. Pure otavite (CdCO3) was recovered in this way, with a proportion of the particles of nanoscale dimensions. Fungal isolates from calcareous soil could precipitate CaCO3 and SrCO3 as well as olekminskite (Sr(Sr,Ca)(CO3)2) and Sr-containing vaterite ((CaxSr1-x)CO3), resulting in almost complete removal of Sr from solution. Metal carbonates have several industrial applications and are also precursors for important metal oxides. In an imaginative demonstration, thermal decomposition of fungal biomass and precipitated MnCO3 resulted in a carbonised biomass-manganese oxide composite material and used as an electrode material in supercapacitors and lithium ion batteries. This was found to have some very good electrochemical properties in comparison with other Mn oxide materials prepared by abiotic means and in lithium ion batteries retained ~90% charge capacity after 200 charge-discharge cycles.
Metal-containing micro-/nanoparticles have a wide variety of applications. The use of metal-transforming microbes for production of nanoparticles may allow some control over size, morphology, and composition. This is relevant to the production of new advanced biomaterials with applications in metal and radionuclide bioremediation, metal biorecovery, antimicrobial treatments (e.g. nano-silver), solar energy, electrical batteries and microelectronics. Many fungi can precipitate nano-elemental forms of metals and metalloids through bioreduction, e.g. Ag(I) reduction to elemental silver Ag(0); selenate [Se(VI)] and selenite [Se(IV)] to elemental selenium [Se(0)]; tellurite [Te(IV)] to elemental tellurium [Te(0)]. Many of the fungal biominerals mentioned previously can be nanoscale or microscale, which imparts additional properties apart from metal sequestration. For example, mycogenic Mn oxides can sequester metals like Pb, Zn, Co, Ni, As and Cr and also oxidize certain organic pollutants. Many fungi produce metal oxalates on interacting with a variety of different metals and metal-bearing minerals, e.g. Ca, Cd, Co, Cu, Mg, Mn, Sr, Zn, Ni and Pb and these have various industrial uses as well as providing another metal biorecovery mechanism.
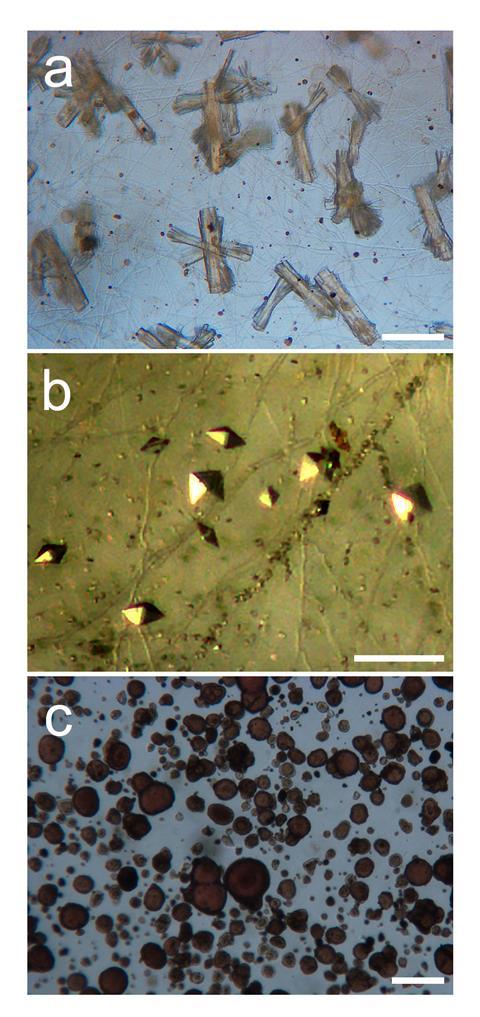
Fungi are ubiquitous in natural environments and their roles in organic matter decomposition, element cycling and plant productivity are profound. As inhabitants of soil and rock surfaces, they are engaged in a suite of metal and mineral transformations altering metal speciation and mobility through such mechanisms as complexation, mineral dissolution and secondary mineral formation. These are also of negative human impact with the same mechanisms being involved in the biodeterioration of rock and mineral-based materials in the built environment and cultural heritage. On the positive side, their interactions with metals and minerals are of applied significance in land bioremediation, revegetation and detoxification of industrial effluents and waste streams, and are an important component of the overall microbial repertoire of mechanisms that are being applied to metal biorecovery and the production of useful biomaterials. With growing concern over the management, conservation and recycling of world metal and mineral resources, it is clear that fungal capabilities may offer potentially useful solutions to an apparently insoluble problem.








No comments yet