In an interdisciplinary approach, research teams from Heidelberg University and Heidelberg University Hospital have investigated the final stages of viral penetration using electron tomography and computer simulations.
In the case of influenza A, they were able to determine how the immune system fights off the virus using a small protein. For Ebola viruses, they discovered that a specific protein structure must be disassembled in order for an infection to take hold.
So-called fusion pores, through which the viral genome is released into the host cell, play a central role in these processes. If they can be prevented from forming, the virus is also blocked. The Heidelberg scientists describe previously unknown mechanisms, which might lead to new approaches to prevent infections.
Lipid membrane
Many viruses that infect humans are covered with a lipid membrane that has glycoproteins that can dock with human cells. In viruses like influenza A, which enter through the respiratory tract, these are the spike proteins that mainly bind to epithelial cells in the nose and lungs.
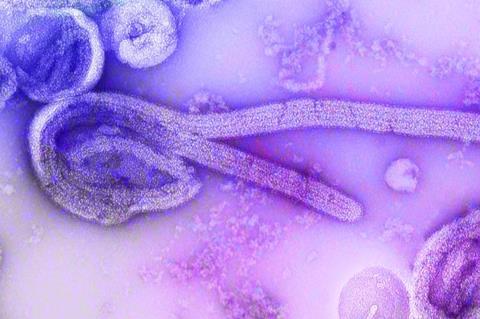
In contrast, the highly infectious Ebola virus spreads through direct contact with infected bodily fluids and can penetrate a broad spectrum of cell types. After invading human cells, these viruses must open a fusion pore between the virus membrane and the host membrane to release their genome into the host cell and propagate.
To fight off the virus, the human immune system attempts to block the formation of the fusion pore in a multi-stage process. Infected cells sense the presence of the foreign genome and send a signal, in the form of an interferon molecule, to as yet uninfected cells.
This signal triggers the uninfected cells to produce a small cellular protein called interferon-induced transmembrane protein 3 (IFITM3).
Specialised protein
“This specialised protein can effectively prevent viruses such as influenza A, SARS-CoV-2, and Ebola from penetrating, but the underlying mechanisms were unknown,” states virologist Dr Petr Chlanda, whose working group belongs to the BioQuant Center of Heidelberg University and the Center for Integrative Infectious Disease Research of Heidelberg University Hospital.
The researchers were now able to demonstrate that for influenza A viruses, IFITM3 selectively sorts the lipids in the membrane locally. This prevents the fusion pores from forming.
“The viruses are literally captured in a lipid trap. Our research indicates that they are ultimately destroyed,” explains Dr Chlanda.
To analyse the structural details of viruses, Dr Chlanda and his team took advantage of equipment from the Cryo-Electron Microscopy Network at Ruperto Carola. In an interdisciplinary approach, the research groups led by Prof. Dr Ulrich Schwarz of the BioQuant-Center and the Institute for Theoretical Physics along with Prof. Dr Walter Nickel of the Heidelberg University Biochemistry Center predicted this process with the aid of computer simulations.
In the context of antiviral therapy, the researchers believe it is possible to develop lipid-sorting peptides that insert themselves into the virus membrane, rendering the viruses incapable of membrane fusion.
“Such peptides could be used in a nasal spray, for example,” saysPetr Chlanda.
Ebola penetration
In a second study, the Heidelberg researchers investigated the penetration and fusion of the Ebola virus. The filamentous morphology of the virus is determined by a flexible protein envelope known as the VP40 matrix protein layer.
“It has always puzzled us how this long virus could penetrate the cell, fuse with the membrane, and release its genome,” says Dr Chlanda.
Using their structural analysis of infected but inactive cells provided by collaborators from the Friedrich Loeffler Institute in Greifswald, the researchers discovered that this virus protein envelope disassembles at a low pH, i.e. in an acidic environment.
This step is not least decisive for the formation of fusion pores, as further computer simulations showed. During this process, the electrostatic interactions of the VP40 matrix with the membrane are weakened, thereby reducing the energy barrier of pore formation.
The results of the Heidelberg basic research suggest that a blockade of the disassembly of this layer would be one way to maintain Ebola viruses in a state that does not permit fusion pore formation. Similar to the influenza A virus, the Ebola virus would then be lured into a trap from which it could not escape.



No comments yet