Methamphetamine (Meth) abuse can cause serious mental disorders, including anxiety and depression. The gut microbiota is a crucial contributor to maintaining host mental health. The authors of this article investigated if microbiota participate in Meth-induced mental disorders, and the potential mechanisms involved.

In a study published in Acta Pharmaceutica Sinica B, 15 mg/kg Meth resulted in anxiety- and depression-like behaviors of mice successfully and suppressed the Sigma-1 receptor (SIGMAR1)/BDNF/TRKB pathway in the hippocampus.
Meanwhile, Meth impaired gut homeostasis by arousing the Toll-like receptor 4 (TLR4)-related colonic inflammation, disturbing the gut microbiome and reducing the microbiota-derived short-chain fatty acids (SCFAs).
Fecal microbiota
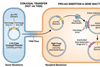
Moreover, fecal microbiota from Meth-administrated mice mediated the colonic inflammation and reproduced anxiety- and depression-like behaviors in recipients. Further, SCFAs supplementation optimized Meth-induced microbial dysbiosis, ameliorated colonic inflammation, and repressed anxiety- and depression-like behaviors.
Finally, Sigmar1 knockout (Sigmar1−/−) repressed the BDNF/TRKB pathway and produced similar behavioral phenotypes with Meth exposure, and eliminated the anti-anxiety and -depression effects of SCFAs. The activation of SIGMAR1 with fluvoxamine attenuated Meth-induced anxiety- and depression-like behaviors.
The findings indicate that gut microbiota-derived SCFAs could optimize gut homeostasis, and ameliorate Meth-induced mental disorders in a SIGMAR1-dependent manner. This study confirms the crucial role of microbiota in Meth-related mental disorders and provides a potential preemptive therapy.
’Gut microbiota-derived short-chain fatty acids ameliorate methamphetamine-induced depression- and anxiety-like behaviors in a Sigmar-1 receptor-dependent manner’ appears in Acta Pharmaceutica Sinica B.





No comments yet