University of Konstanz biologists have discovered a phosphorus-based bacterial metabolism that is both new and ancient, thanks to a calculation from the 1980s, a sewage plant, a new bacterial organism, and a remnant from around 2.5 billion years ago.

The story begins at the end of the 1980s, with a sheet of paper. On this sheet, Bernhard Schink, now professor at the Limnological Institute of the University of Konstanz, calculated that the conversion of the chemical compound phosphite to phosphate would release enough energy to produce the cell’s energy carrier – the ATP molecule.
It should therefore be possible for a microorganism to supply itself with energy. Unlike most living organisms on our planet, this organism would not be dependent on energy supply from light or from the decomposition of organic matter.
Key enzyme unknown
Schink actually succeeded in isolating such a microorganism from the environment. Its energy metabolism is based on the oxidation of phosphite to phosphate, just as predicted by the calculation. However, the key enzyme needed to understand the biochemistry behind the process remained hidden – and thus the mystery remained unsolved for many years.
But three decades after he made the calculation on paper, an unexpected discovery set the ball rolling again. What had been in the back of his mind for many years was finally found in, of all places, a sewage plant in Konstanz, only a few kilometres from Bernhard Schink’s laboratory.
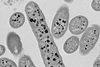
Zhuqing Mao, a biology doctoral researcher from Konstanz, examined a sewage sludge sample and discovered a second microorganism that also gets its energy from phosphite. The Konstanz biologists led by Bernhard Schink placed this bacterium in an environment in which it had only phosphite as a food source and the bacterial population grew.
Autotrophic organism
“This bacterium subsists on phosphite oxidation, and as far as we know, exclusively on this reaction. It covers its energy metabolism this way, and can build up its cell substance from CO2 at the same time,” explains Schink. “This bacterium is an autotrophic organism, like a plant. It does, however, not need light like a plant, as it draws its energy from phosphite oxidation”.
Surprisingly, it turned out that the bacterium is not only a new species, but actually forms an entirely new genus of bacteria.
From that point on, things happened very quickly. A whole network of Konstanz researchers dedicated themselves to unravelling the mystery, including Bernhard Schink, Nicolai Müller, David Schleheck, Jennifer Fleming and Olga Mayans. They produced a pure culture of this new bacterial strain, in which they were finally able to identify the key enzyme that triggers the oxidation of phosphite to phosphate.
Breakthrough experiments
“The breakthrough came with Nicolai Müller and his enzyme experiments,” says David Schleheck. Nicolai Müller succeeded in clearly demonstrating the enzyme’s activity, thereby uncovering the biochemical mechanism behind the key enzyme. Olga Mayans and Jennifer Fleming created a three-dimensional model of its enzyme structure and active centre to understand the reaction pathway.
“What was very surprising was that during its oxidation, phosphite is apparently coupled directly to the energy-carrier precursor AMP, whereby the energy carrier ADP is created. In a subsequent reaction, two of the generated ADPs are converted to one ATP, on which the organism ultimately lives,” Nicolai Müller outlines the reaction pathway.
The discovery of this new type of energy metabolism is outlined in a paper in PNAS. The research team thinks that this type of metabolism is by no means new, but very old, even ancient: around 2.5 billion years old.
Early days
“It is assumed that in the early days of evolution, when the Earth was cooling down, phosphorus was still present to a large extent in a partially reduced form and was only later gradually oxidized. The metabolism we have now discovered fits very well into the early phase of the evolution of microorganisms,” Bernhard Schink explains.
The biochemical mechanism that the bacterium uses for its metabolism is therefore not new, but has most probably been preserved from the primeval times of our planet: back when life on our planet began and the first microorganisms had to feed on inorganic compounds such as phosphite.
Thus the new scientific findings provide clues to the early biochemical evolution on our planet, and provide the key to a biochemical mechanism that makes life possible in very hostile places, possibly even on alien planets.





No comments yet