Recent advancements in flexible bioelectronics have demonstrated remarkable progress in achieving seamless integration at bio-tissue-electronic interfaces. However, persistent challenges such as foreign body response (FBR) due to mechanical mismatch and signal instability under dynamic physiological conditions remain critical barriers.
“By synergizing bioinspired chemical modifications with microstructural topology, we developed a self-healing bioadhesive interface that eliminates reliance on external stimuli, overcoming the physiological incompatibility of traditional rigid encapsulation materials,” emphasized corresponding author Professor Ming Wang (Fudan University).
READ MORE: Using living bioelectronics to treat chronic inflammation
READ MORE: New method developed to dramatically enhance bioelectronic sensors
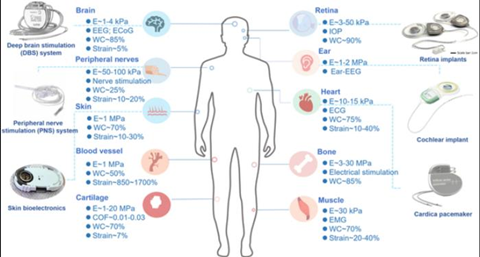
The proposed three-layer material architecture integrates: (a) mussel-inspired catechol-functionalized polyurethane elastomer substrates with brain tissue-like modulus (<1 kPa); (b) conductive hydrogels crosslinked by dynamic borate ester bonds, exhibiting ultrahigh toughness (420 MJ/m³); and (c) MXene-silk fibroin composite anti-inflammatory coatings that suppress immune responses via reactive oxygen species (ROS) scavenging.
“This multiscale design actively modulates macrophage polarization during early implantation and restores 90% conductivity within 48 hours post-mechanical damage,” added lead author Dr. Xiaojun Wu.
Signal acquisition
Validation through rat cortical implantation experiments demonstrated stable electrophysiological signal acquisition over 30 days (signal-to-noise ratio (SNR) of 37 dB vs. 15 dB for conventional Pt electrodes), with fibrous capsule thickness reduced to one-third of traditional materials (28.6 ± 5.4 μm vs. 85.2 ± 12.7 μm).
The self-healing hydrogel maintained a conductivity of 1.2 S/cm under 100% tensile strain, showing only an 8.7% impedance increase after 10,000 mechanical cycles. Compared to commercial silicon-based Utah arrays, the system improved motion artifact suppression by 40% (RMS error <15 μV) and achieved synchronized drug release (82% cumulative release over 72 hours).
“Microfluidics-assisted 3D printing enabled precise fabrication of vascularized conductive networks, achieving a curvature adaptation radius of 200 μm,” highlighted Wang. Current limitations include a 23% conductivity decay after 28 days of biofluid immersion, prompting future plans to introduce biomimetic mineralization layers to enhance barrier functionality. This work pioneers the integration of material chemistry intelligence with bioelectronic functionality, establishing a novel paradigm for adaptive neural interfaces and advancing the development of diagnostic-therapeutic implantable systems.
Authors of the paper include Xiaojun Wu, Yuanming Ye, Mubai Sun, Yongfeng Mei, Bowen Ji, Ming Wang, and Enming Song.






No comments yet