Over the last 10–15 years, the human microbiome has emerged as a central player in human health and well-being. The study of these microbes is moving forward at breakneck speed, with scientists probing their composition and function, to develop new therapies that have a wide range of disease targets.
Over the last 10–15 years, the human microbiome has emerged as a central player in human health and well-being. The study of these microbes is moving forward at breakneck speed, with scientists probing their composition and function, to develop new therapies that have a wide range of disease targets. Importantly, ‘first contact’ between microbes and their host represents a critical developmental window in which the foundations for lifelong health are laid down. Indeed, it is now recognised that disturbing this fledgling microbial ecosystem has both short- and long-term consequences, with microbiota ‘dysbiosis’ linked to increased incidence of diseases including obesity, asthma, inflammatory bowel disease (IBD) and certain brain conditions. Thus, understanding the factors that modulate the microbiome during the first stages of life, during pregnancy and infancy, is a key focus for numerous groups, including our own, which is underpinned by large-scale human cohort studies alongside key mechanistic experiments.
Microbial discovery
Our significant steps forward in microbiome research have come hand in hand with ongoing technological development. The majority of recent studies have utilized detection methods based on DNA/RNA sequencing, which has enabled us to uncover the ‘black box’ of microbial complexity and has greatly aided in microbial identification and functional profiling. Although bacteria have been the major focus, more recent studies have started to profile other key microbes including archaea, fungi and viruses. Research groups have also been developing improved culturing methodologies to isolate and further characterize our resident microbes, which is a critical requirement for therapy development and mechanistic insights. Indeed, as a field, we are beginning to move away from ‘who is there’ to ‘what can they do,’ using more functional readouts including transcriptomics (both host and microbes), and metabolite profiling, in tandem with innovative in vitro and in vivo models to probe causal insights gained from human studies.
Current understanding of the microbiota during pregnancy
Profiling the maternal microbiome over the course of pregnancy indicates a change in communities in preparedness for birth, and coincides with hormonal, immunological and metabolic gestational changes. In the gut, previous studies have indicated an increase in diversity (including an increase in Actinobacteria), particularly during the third trimester, which contrasts with vaginal communities, which appear to have a reduction in overall diversity, with Lactobacillus dominating. Importantly, diet and other factors such as antibiotics have been implicated in microbial perturbations during pregnancy and may impact pregnancy outcomes; for example, antibiotic use during pregnancy has been linked to 25% of premature deliveries. More work is required in this area to unravel the numerous factors that impact the maternal microbiome and what this means for birth and microbial seeding of infants.
For many years, the foetus and womb were considered sterile until after birth, when initial microbial colonization begins. However, recent work suggests that microbes may be present at these very first life stages, including a placental microbiome, although this is somewhat controversial. Whereas whole microbes in utero have been associated with negative pregnancy outcomes, including preterm birth, it does appear that microbial products may cross to the developing foetus; thus, initiating the first microbe–host cross talk.
Composition and role of early life microbiota during infancy
Regardless of potential in utero seeding, establishment of our microbial–host symbiotic relationship starts at birth after massive microbial exposure. So where do babies get these initial microbial pioneers from? Vaginal, skin and gut-associated microbes transfer to infants during childbirth, with birth mode having a significant impact on initial colonization. C-section infants show intestinal bacterial composition similar to the skin microbiome (e.g., Staphylococcus, Corynebacterium), and higher levels of hospital-associated microbes. Contrastingly, babies born vaginally are colonized by facultative anaerobes including Lactobacillus, which within the first few days of life reduce the oxygen rich (i.e., aerobic) new-born gut to allow oxygen-sensitive anaerobes such as Bifidobacterium spp. to colonize. It appears that this initial microbial dosing impacts the overall microbial composition, particularly in the short term, which also represents a critical time for immune programming. Notably, previous studies have linked C-section delivery with increased risk of allergic-type diseases, including asthma and atopic dermatitis, and as such there has been an increasing interest in vaginal seeding. This involves coating new-born C-section babies in vaginal fluid from the mother to allow the transfer of microbes. Although this research is at initial stages, it has already been widely taken up in certain countries including Australia, whereas other countries like the UK still have concerns with the transfer of potentially pathogenic microbes, such as Group B Streptococcus.
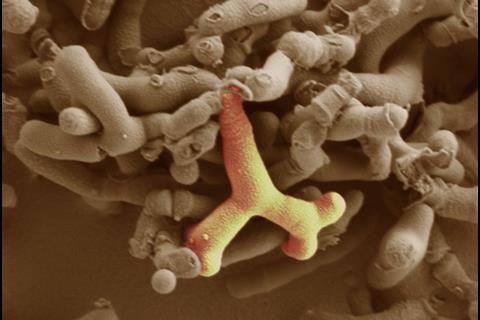
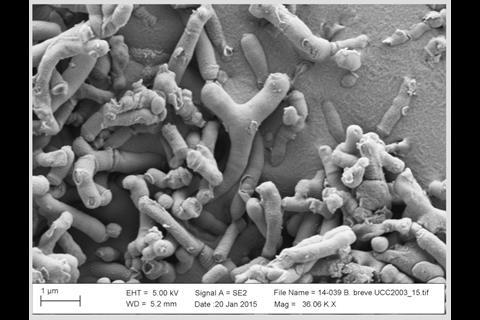
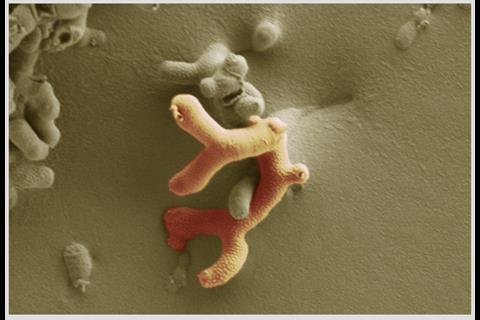
The early life microbiota is in constant flux with diversity and richness increasing over time, until 2–3 years of age when we reach a relatively stable adult ‘climax’ community. Contrastingly, alongside enhanced bacterial diversity, a contraction in the virome, i.e., the bacteriophage community, is observed. Arguably the strongest influencing factor on microbiota composition during this time is diet. Strong differences are observed between breast- and formula-fed infants, with a dominant Bifidobacterium composition in response to breast feeding, while bottle feeding supports wider diversity (including more Enterobacteriaceae), and a reduction in bifidobacteria levels. The components of breast milk, including human milk oligosaccharides (HMOs), are preferentially metabolized by bifidobacteria, thus enabling them to outcompete other microbes. Currently, several groups, including our own, are seeking to determine the molecular factors that help bifidobacteria break down breast milk components, with the aim of developing new infant formulas.
On average, infants receive ~3 courses of antibiotics before their first birthday. As the early life ecosystem is just finding its feet, this represents a key period in which antibiotics may negatively impact microbial communities. Antibiotics are critical for fighting off serious bacterial pathogens, but they do not discriminate between bad and beneficial bacteria, and numerous studies have indicated that antibiotic use in early life strongly reduces microbiota diversity and may lead to ‘extinction’ events. Large-scale epidemiology studies have linked antibiotic use with increasing incidence of chronic gut diseases including IBD, Crohn’s disease and ulcerative colitis, with some reports suggesting that successive antibiotic courses during the first year of life increase IBD risk over seven-fold. Bifidobacterium communities are often severely impacted after antibiotic usage (as they are rarely antimicrobial resistant) and our ongoing studies indicate that a variety of bifidobacterial species and strains strongly influence immune development, including strengthening of the gut epithelial barrier, which is ‘leaky’ in IBD patients. Thus, exploring how these microbes play a beneficial role in intestinal health is crucial if we are to develop and translate novel microbial-associated therapies into the clinic.
Ecosystem restoration
There are currently several ecosystem restoration strategies available, including the radical faecal microbiota transplant (FMT) approach. Patients who have Clostridium difficile infection, and for whom antibiotic treatment has been unsuccessful, are now able to get FMT on the NHS, although not all hospitals are able to offer this as standard care due to the requirement for close collaboration with microbiology groups based at universities and research institutes. This treatment was so successful, with a 94% cure rate, that it was fast tracked onto The National Institute for Health and Care Excellence (NICE) guidelines. More recently, ‘filtered FMT,’ leaving only small metabolites and bacteriophage, has also been shown to successfully treat C. difficile infection. Various companies are now working towards a more refined FMT approach with defined and standardized microbial communities for the treatment of infections and more complex diseases such as IBD. In infants, rather than FMT (although a 13-month-old patient has been successfully treated), a simpler approach is often used, including the administration of probiotics, defined by the WHO as ‘live organisms which, when administered in adequate amounts, confer a health benefit on the host.’ Traditionally, species and strains belonging to the Lactobacillus and Bifidobacterium genera have been used to prevent or treat a variety of diseases, but at this time there are no fully licensed ‘probiotics’ on the market, as the scientific evidence, as deemed by the European Food Safety Authority (EFSA), is not robust enough. Groups, like our own, are working towards more rational design of Bifidobacterium therapies, which are Generally Recognized As Safe (GRAS) microorganisms. Using a combination of genomics, molecular microbiology, model colon systems and innovative preclinical models including germ-free animals, we are aiming to translate these therapies to improve maternal and infant health. Indeed, we have an ongoing study in preterm infants that is seeking to reduce disease burden in these at-risk babies via supplementation with Bifidobacterium.
As microbiologists we realize the importance of keeping our resident microbes happy and healthy, as this directly relates to our well-being. C-section births are on the rise, antibiotic use is frequent, which also links to increasing antimicrobial resistance (AMR), and our diet is changing. This correlates with increasing incidence of allergic, metabolic and chronic diseases that place a significant burden on healthcare systems, including the NHS. To maintain, or re-wild disturbed ecosystems, particularly during the first stages of life, represents an important and exciting area of study. Advancements in our understanding of the host–microbe interaction during this crucial time, provides us with a motivating outlook to the future, moving us closer to our goal of lifelong health.
To find out more about Dr Lindsay Hall’s research please visit www.halllab.co.uk










No comments yet