Plastic materials have been around for a while. For example, PVC was invented in 1920 and nylon stockings became all the rage in the 1940’s due to their function, durability and affordability. But it was not until post WWII that plastic entered the mainstream of consumerism. First reported in the marine environment about 50 years ago, plastic is now estimated at 150 million metric tonnes with 30 MT entering the ocean environment each year.
The Plastisphere
Highly engineered for properties such as line-speed for industrial packaging, shine, colour, durability, and resistance to air-permeability, most plastics are intended for single-use and are blended with proprietary additives and or co-polymers that make them difficult to recycle. Plastic material engineering has yet to prioritise recyclability and reuse over properties driven by initial user demand– leading much of plastic material (about 70% of plastics are single use) to be released into the environment, pulsing into watersheds with storm events, and ultimately, the oceans. Once in the oceans, plastic can transport into the cores of our oceans, known as gyres, bordered by currents with no escape, where these polymer materials can fragment from wave action, UV irradiation, and other yet to be quantified effects from halogen ions and microbially-mediated oxidation.
A decade ago, colleagues and I described a new habitat created by humans, the Plastisphere, the surface of plastics in the natural environment. This new habitat is unlike any other natural habitat by metrics of hydrophobicity, durability, and long-residence times—in other words, the same properties that made plastic a favourable material make it a formidable one in the environment. Plastic moves like surface prey, attracting seabirds, and even more diabolically, smells like food due to the algal component of its microbiome. Lower density plastics (HDPE, LDPE, PP, foamed PS among others) in the size range >300 micron tend to float or remain in the upper 30 meters or so of aquatic environments, interacting with biota in ways still being deciphered. Constantly off-gassing, plastics emit a characteristic volatile ‘scent’ that has been shown to be metabolised by microbes. Whether this is a chemotactic, attractive response is yet to be shown, but in our lab, we have observed microbes settling onto plastic surfaces in a matter of minutes
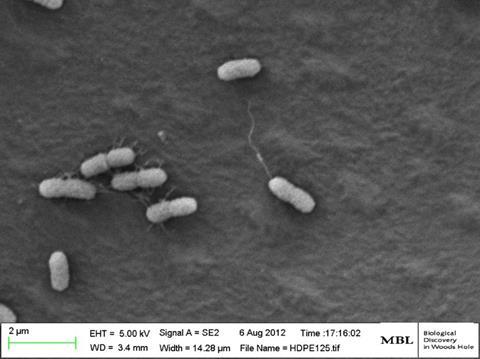
Since then, we have investigated biogeography of microbial communities on microplastics, and the fate of plastics in the marine environment. Scanning Electron Microscopy (SEM) and Confocal Microscopy work by our group and collaborators has shown that once the plastic surface is coated by microbes over a time span of week to months, it looks like any other surface in the ocean, and the rind of microbial film that covers it has a ‘carrying capacity’ that can harbour a surprising amount of life – in the open ocean where surfaces are rare, this can be up to 1% of the estimated total microbial population of the sea surface microlayer. This might not sound like much, but it corresponds to about 10,000 tonnes of microbial carbon biomass!
However, the early stages of microbial settlement, when a plastic surface first contacts the ocean, turned out to be more specific than we expected. It was not until we started experimenting with how microbes settle on virgin plastic surfaces in the short-term (hours or days, as opposed to weeks and months) that we began to realise there may be more specific molecular mechanisms at play.
Incubating various plastic samples along a transect from water off The Bahamas to Chesapeake Bay we found, repeatedly, that petroleum-based plastics attracted a specific group of Alteromonadaceae bacteria containing the mannose-sensitive hemagglutinin (MSHA) Type IV attachment factor genes, first described as essential pathogenicity factors in Vibrio cholerae. Interestingly, the bioplastic polymer polyhydroxyalkanoate (having much more hydrophilic properties than PP, PE and PS petroleum-based polymers used in our study) attracted different microbes devoid of the MSHA operon. The MSHA operon was further implicated in a study of ours focusing on Vibrio spp. We found multiple operons of the MSHA and multiple copies of the mshA gene in these potentially pathogenic Vibrio spp. isolates. These vibrios were isolated from seawater, Sargassum seaweed, plastic debris and eel larvae and all contained multiple copies of the mshA gene, previously shown to increase adhesion, DNA uptake and virulence in V. cholerae. Moreover, these mshA genes appear to be under positive selection, together with other gastrointestinal pathogenic factors such as the Zonula occludens toxin (Zot), known for weakening the tight junctions between intestinal cells in vertebrates. It has yet to be proven but this could be key to the natural history of how these open ocean vibrios hitch a ride into the gut environment of their hosts, liberating nutrients, producing progeny and releasing nutrients to the surrounding oligotrophic seawater.
Other applications
An avenue our group is now pursuing is to understand the purpose and possible applications of the MSHA adhesion system and its potential for biotechnology. Is this adhesion mechanism tuneable to different surfaces such as cell types? Could it be used in microbial therapeutics? Is this the mechanism by which specific foodborne pathogens stick to specific areas in manufacturing and processing pipelines? Certainly, the MSHA has been shown to be common in uropathogens in plastic implantable devices such as catheters. How can we inhibit MSHA adhesion mechanisms? Looking ahead, it is clear that the MSHA adhesion system deserves more attention in biomedicine and in the world around us.







No comments yet