We understand the water cycle and the flow of nutrients in ecological systems, but might microbial life also follow a cyclical, interconnected pattern, and how does that look with regards to food production?
Microbes connect all aspects of the farm environment, from animals to soil to plants to manure, and play a critical role in nutrient turnover, animal health, and environmental quality. This microbial connectivity raises important questions about whether there are measurable patterns in microbial communities that reflect the overall health and safety of agricultural systems. Understanding these patterns is especially important in the context of food safety, as disruptions to microbial balance can influence pathogen dynamics and contamination risks. Research in this area increasingly uses sequencing and bioinformatic tools to map and analyze microbial signatures across different environments, with the goal of informing sustainable practices that promote productivity and public health.
Microbes are not just everywhere; they are essential
Although invisible to the naked eye, microorganisms inhibit every component of the agricultural landscape (farm-scape). They are associated with livestock, present in raw milk, abundant in manure, embedded with soil matrices, colonizing plants above and below ground, and even dispersed through the air. These complex communities, consisting of bacteria (prokaryotes), archaea (eukaryotes), fungi, and even viruses, drive essential processes including the digestion of human-indigestible feeds in the cow gut, nutrient recycling, organic matter decomposition, and pathogen suppression. They do this through a dynamic web of interconnectivity, making them integral to the productivity, resilience, and sustainability of the farm-scape.
Much like the biodiversity in natural ecosystems, the composition and functional potential of these microbial communities can serve as sensitive indicators of animal and soil health, offering potential early-warning signs and opportunities for intervention. However, agricultural practices such as dietary formulation, tillage intensity, crop rotations, and manure processing and application significantly influence the microbial community’s structure and stability. A deeper understanding of how these practices shape microbial networks can support more informed decision-making, allowing farmers to manage their land and animals more sustainably while safeguarding environmental and food safety outcomes.
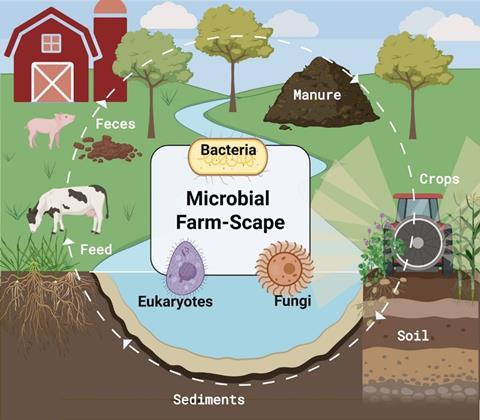
Microbial communities on farms have effects that extend far beyond the farm gate, intersecting with broader public health issues such as food safety and antibiotic resistance. In recent years, the “One Health” concept has gained traction, emphasizing the interconnectivity of animal, environmental, and human health. For example, antimicrobial resistance (AMR) is not confined to a single sector (i.e. human or animal), but is a shared challenge that demands a coordinated, cross-sectoral response.
Addressing AMR effectively requires recognizing its global dimensions and the complex interplay of anthropogenic, zoogenic, ecological, and even climatic factors that shape health outcomes on a planetary scale. In 2019, AMR was directly responsible for 1.27 million global deaths and contributed to 4.95 million deaths (Antimicrobial Resistance Collaborators, 2022). While extensive efforts are being directed towards a better understanding of AMR and its transmission, the main focus has been set on human health. While some studies have been conducted under specific animal husbandry settings, antimicrobial-resistant bacteria are found across all types of farm environments, even in antibiotic-free farms (Langlois et al. 2025), meaning that other factors that contribute to their presence and potential transmission remain unknown. Therefore, to develop effective One Health solutions, it is critical to first understand the dynamics within farm ecosystems (animals and environment) and how these microbial interactions could have potential impacts on human health.

What is at stake on farms?
Nutrients contained in animal diets are not 100% absorbed by the animal, meaning that nutrients (nitrogen, phosphorus, etc.) and other unabsorbed trace metal elements (TME), as well as a variety of microbes are excreted from the animals, and are found in the effluents (Marchand et al. 2024). In agriculture, these effluents are used as crop fertilizer based on their nutrient properties and are an important source of soil nutrients on farms with animal production.
However, often the application rate of livestock effluents is set according to their phosphorus concentration, but no restriction is imposed on other components, such as microbes or TME, that they can bring to the soil. One of the major concerns currently is the bioaccumulation of TME inputs from livestock manure, which exceed plant requirements and remain in the soil year after year, with the potential to enter the water table.
Why is this a concern? Previous research has shown that multi-year manure application can increase the TME content of soils (Brock, 2006), which is a major concern in areas where livestock and crop production border protected ecosystems or urban areas. This could then result in the exposure of these areas to toxic levels of heavy metals or the transfer of biological contaminants via various vectors, including water and direct or indirect human transmission. Furthermore, it is becoming apparent that there is the potential for the co-selection of antibiotic and metal resistance genes in association with TME, such as zinc, copper, cobalt, and other minerals (Li et al. 2017).
While the understanding of these processes can be tested in a lab, real-time evaluation and a better understanding of the mechanistic influences of TME on the microbial farm-scape requires analysis on a genetic level, to determine the presence and absence of a variety of molecular components. These can be detected on-farm, simultaneously, and across a vast majority of environmental influences, such as season, temperature, and the various microbial kingdoms, including fungi and eukaryotes.
Genetic content moves across the farm-scape
The transfer of genetic content across various niches of a farm ecosystem is understood especially in relation to human contact. The transfer of pathogens and their genetic content through direct contact between humans and animals, between contaminated material and human footwear, form the basis of understanding for current rules and regulations on farm biosecurity, and cleanliness protocols for farm visitors such as veterinarians and nutritionists that regularly travel between farms.
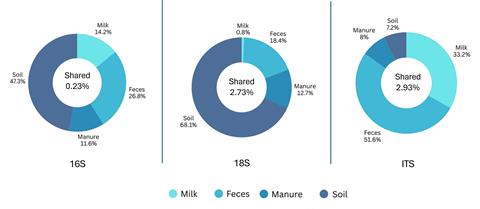
But the impact on the environment (soil, sediment, water) is less clear, and more specifically, how similar these niches are in terms of genetic overlap. Since the microbiome complexity of environmental samples is high, it requires assessment using molecular tools, including sequencing and bioinformatic comparison, to be able to assemble the big picture regarding the various taxonomic kingdoms. By using genetic targets to analyze the same farm sample for bacteria (16S), eukaryotes (18S), and fungi (ITS), a much more complex picture of the ecological web can be built.
Ricci et al., (2025) showed that despite seasonal variation in sampling (spring versus winter) and various impacts of temperature, genetic content was shared across the farm-scape from dairy cow raw milk samples, to feces, to stored manure, and finally to the soil where animal feed was grown (shown below).
”Interestingly, while most AMR research emphasizes the transmission of clinically relevant human bacterial pathogens, in the animal-environment interface of farm-associated One Health, it is fungal populations that share a higher proportion of genetic similarity across biological samples.”
These observations underscore the potential role of fungal contamination and persistence in the farm-scape. This aligns with the growing international concern, as reflected in the World Health Organization’s development of a Fungal Priority Pathogens List, which highlights the urgent need to address fungal threats in both human, animal, and environmental health contexts.
While ecosystem-wide sequencing provides a valuable insight into the structure and dynamics of microbial communities, a major limitation is in understanding whether the genetic content of these samples was part of a viable and growing population or just the remnants of material transferred from other niches. Therefore, it is equally critical to identify and track specific pathogens that are known to impact both animal and human health.
Integrating molecular techniques with microbial growth assays ensures that One Health research captures the complexity and the direct risks to public health. Recent assessments of viable Enterobacteriaceae and Enterococcus showed contamination across feed, feces, manure, crop soil, forest soil, surface water, and sediments using classical microbiology tests (shown below). Enterobacteriaceae resistant to either cefotaxime or ciprofloxacin were recovered from all tested matrices, whereas vancomycin-resistant Enterococcus were found only in some pig feed, feces, and manure samples. The presence of these pathogens indicated that resistant bacteria can be found throughout the farm-scape, even in forest soil and surface water upstream of the crop soil, suggesting widespread environmental reservoirs. The presence of Enterococcus in soils associated with food crops is influenced by manure application.
By clarifying these interactions between sources, it will be possible to add additional factors that could alter transmission patterns in different parts of the globe. For example, the winter freeze and spring melt of northern areas, such as in Canada, impact mobilization and persistence of both the genetic material from microbial communities, the overall community diversity, and also the persistence of resistant bacteria across the farm-scape. This information can then be applied to models for the prediction of AMR flow and the design of mitigation strategies that reflect anthropogenic inputs and natural ecological dynamics.
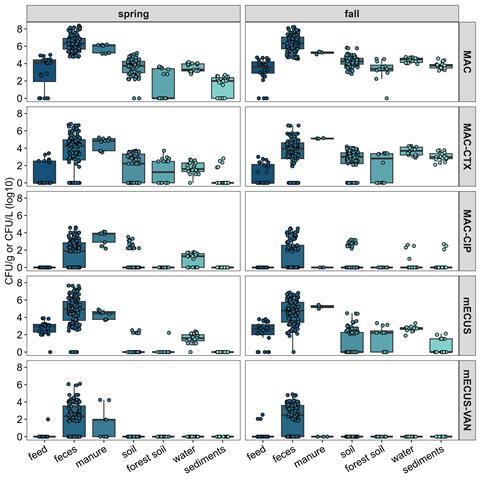
A key component of the farm-scape model is also understanding the role of antibiotic usage in the development and transmission of resistance. Even antibiotic-free animals, raised on antibiotic-free farms, host antibiotic-resistant bacteria in their guts. Resistant bacteria were recovered from both feces and the surrounding environment, not only downstream of the studied fields. This suggests that other factors, other than the use of antibiotics in animal production, contribute to their presence.
These findings highlight the need to distinguish between AMR originating from livestock management factors and those arising from natural environmental reservoirs. Livestock factors can include vertical transmission of bacteria from the mother to offspring at birth, supranutritional feeding of TME, or even the use of other antimicrobials for cleaning and disinfection. Whereas, in the environment, while resistant bacteria can be naturally found due to environmental selection pressures, wildlife can also account for their presence and transmission.
An important insight emerging in this field is that manure serves not only as a nutrient-rich fertilizer, but also as a significant vector for microbial dissemination across the farm-scape. Fungi appeared to be most consistently transmitted, suggesting that they may play a more prominent role in the farm-to-food web as previously recognized. However, this work needs to be validated through culture assays to validate the types of fungi and their potential threats to the One Health network.
Why it matters
Studying AMR in the farm-scape is relevant to enhance manure management policies. The current policies regarding manure application in some countries regulate the level of specific nutrients, like phosphorus and nitrogen, but do not take into account the microbial or AMR load, while manure can act as a vehicle for transmission of microbes, specifically fungi. Looking at the whole farm-scape instead of focusing on only feces or manure is also meaningful to identify hotspots of AMR. Once hotspots are known, concrete solutions can be suggested and applied to either decrease their level at this specific point or limit their transmission across the farm-scape. For example, some have suggested manure composting to decrease the level of pathogens and AMR load (Marutescu et al. 2022). Respecting the riparian zone, which is a 10 to 15 m zone between agricultural fields and a water body, can also limit the contamination of water streams by manure effluents. However, while regular surveillance of AMR is critical, so is the surveillance of the microbial community as a whole. Fungi and eukaryotes are often overlooked compared to bacteria, but can also be an important contamination source as they, too, can move around the farm-scape.
Despite their microscopic size, microbes exert influence far beyond the farm gates, reaching into our food systems and the communities downstream. Nutrients, air, and water link environmental, animal, and human health, creating connections between the core pillars of the One Health framework. As such, given the ubiquity and complexity of microbial life, a narrow focus on a single matrix, season, or pathogen only offers a partial view. Integration of microbiology and bioinformatics with ongoing surveillance efforts is essential to capturing the full scope of microbial interactions while minimizing future threats to public health.








No comments yet