During his journey on the Beagle, Darwin observed microbial life in an Argentinean salt pond, and then envisioned an array of other extreme environments where life was likely to be present: on mountain peaks, at the poles, in volcanoes, below the ocean depths and in the deep subsurface. Over time all but the last were proven right. Deep subsurface life has been the most refractory because it has taken almost two hundred years to demonstrate that he was right.
Continental deep subsurface geomicrobiology is essential to our understanding of the importance of Earth’s biogeochemical cycles at a planetary scale. It has been estimated that most of the prokaryotic biodiversity and biomass of our planet is under our feet, but we have limited information on who is down there, and most importantly of all, of how biogeochemical cycles operate in the so-called dark biosphere. Thus, the geomicrobiological characterisation of the deep subsurface is considered a new frontier in science and an important tool with which to understand the operation of our planet. In addition, this type of lifestyle has important astrobiological connotations for the existence of life on Mars and other planetary bodies.
Some pioneering observations have been made in oil field waters, but the general opinion is that the detected microorganisms were the product of contamination during the drilling operations. This is still the main problem associated with the geomicrobiological characterisation of the deep subsurface, in which appropriate controls are required to rule out the possibility that the detected microorganisms are the product of contamination. After the very successful campaigns to study the deep oceanic subsurface, mainly in sediments, geomicrobiologists have started to wonder if life could thrive in the continental deep subsurface. The first approaches used artesian wells and groundwater obtained from the walls of deep mining operations. Despite providing useful information on microbiological diversity, these cannot be directly related to the pertinent geological features of the complex solid matrix which the microorganisms inhabit. Despite recent progress in the field, information concerning the diversity, abundance, ecophysiology, as well as the biogeochemical cycles operating in the continental subsurface hard rock ecosystems is still scarce.

To generate information from the deep subsurface, devoted drilling is required to generate core samples with which to perform essential complementary analysis. The few devoted continental drilling operations carried out to date have reported, in general, microbial diversity from samples obtained at great depths and use just one methodology, generally sequencing, which does not produce sufficient reliable information to accurately describe the operation of the biogeochemical cycles in the deep subsurface.
To overcome these limitations, we recently published a detailed multi-methodological analysis on the microbiology and microbial driven geochemical processes in the deep subsurface rock matrix of the Iberian Pyrite Belt (IPB). A total of 47 samples obtained in sterile and strictly anaerobic conditions at different depths from cores of a 612m borehole were analysed using different complementary methodologies (massive sequencing, cloning, immunological detection, fluorescence-in-situ-hybridisation, enrichment cultures and isolation). The results obtained allowed identification of the most representative microorganisms involved in the light independent, coupled C, H, N, S and Fe biogeochemical cycles operating in the deep subsurface of the IPB. Enrichment cultures were used to identify the most characteristic anaerobic metabolic activities operating in the ecosystem. Twenty-nine microorganisms were isolated from these enrichment cultures and are being characterised phenotypically and genotypically. The genomic analysis of nine of these isolates allowed the identification of genes involved in the operation of the biogeochemical cycles and highlighted the role of the nitrogen cycle and in particular, the nitrate-reducing microorganisms in the subsurface of the IPB.

Of the different methodologies used in this work, fluorescence-in-situ-hybridisation (FISH and CARD-FISH) stands out because it not only allowed us to identify the presence of microorganisms but also to quantify them and detect their close contact with other microorganisms, suggesting the functional operation of complementary metabolic activities. In this work we detected the presence of anaerobic ammonia (NH4+)-oxidising bacteria (ANAMMOX); the co-occurrence of archaeal ANME-2 and Sulfate Reducing Bacteria (SRB) (which could explain the methanotrophic activities detected along the borehole); the co-occurrence of Fe oxidising and reducing microorganisms suggesting the operation of an Fe cycle, as well as; Sulphur oxidising and reducing microorganisms suggesting the operation of an S cycle. To the best of our knowledge this is the first time that these metabolic activities have been identified in continental hard rock samples from the deep subsurface.
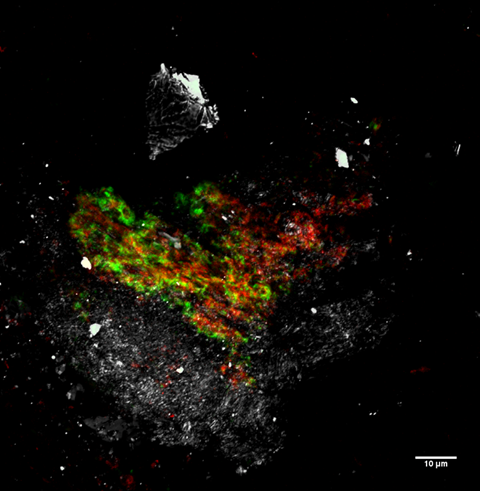
Due to the methodology used, fixation and denaturation of samples immediately after their extraction from within the cores, contamination is very unlikely and impossible to detect by fluorescence-in-situ-hybridisation. In addition, an important advantage over the rest of the methodologies used is that it only requires a very small amount of sample, generating information on the presence of different microorganisms at microscopy size resolution, which cannot be obtained with the rest of the methodologies, as they require larger amounts of sample for their analysis. In the case of solid rock matrices, a larger amount of sample is required to obtain analysable DNA – in our case up to 10g of core sample. Furthermore, fluorescence-in-situ-hybridisation is a non-destructive methodology allowing the same sample to be re-hybridised many times using complementary probes. This facilitates the identification of the diverse microbial components of the sample. Except for fluorescence-in-situ-hybridisation, the other methodologies used yielded interesting diversity results but provided no information on the interconnection between the identified microorganisms and the mineral features of the complex matrix in which they inhabit.
The positive signals obtained using fluorescence-in-situ-hybridisation strongly suggest the presence of metabolically active microorganisms in the deep subsurface, a fundamental question in subsurface geomicrobiology. Although, in this work, most of the microbial identification has been done using CARD-FISH, to amplify the hybridisation signal and facilitate the distinction between real hybridisations and artifacts, such as the presence of auto-fluorescent minerals or the unspecific binding of the probe or dye to the diverse mineral content present in each sample. In addition, the use of fluorescence-in-situ-hybridisation demonstrated the existence of biofilms made of exopolysaccharides in the oligotrophic deep subsurface of the IPB, contrary to the generally accepted idea that in these extreme conditions, microorganisms are unable to use their limited source of energy in the generation of metabolically expensive structures. From our data, we strongly support the idea that, if needed, microorganisms will use their limited resources to make biofilms, taking advantage of their useful properties such as the ability to interconnect metabolically complementary functional microorganisms, protect them against desiccation, control metabolic product diffusion, etc.
As observed in other continental drilling operations, bacteria are much more abundant than archaea in the deep subsurface of the IPB, which seems to be a common property of the continental deep subsurface. We did not observe any depth-related patterns of distribution of functional groups along the column, at least along the 612m analysed. Despite the difficulties in assessing cell density within a solid, low-porosity rock and variable mineralogical content, the analysis of different samples along the borehole yielded values between 104 and 105 cells/gram of rock sample. This is in the same order of magnitude that has been reported in other continental hard rock drilling operations.
In this work we introduced the condition of using complementary methodologies to identify the most relevant microorganisms operating at different depths to mitigate the bias of each methodology. Sixteen genera belonging to the Pseudomonadota (Acidiphilium, Acidovorax, Acidithiobacillus, Brevundimonas, Desulfovibrio, Pseudomonas, Rhizobium, Rhodoplanes, Shewanella), Actinomycetota (Arthrobacter, Propionibacterium, Tesaracoccus), Bacillota (Bacillus, Desulfosporosinus, Sulfobacillus) and Nitrospirota (Leptospirillum) phyla and members of the class Cyanobacteria, were selected because they had been detected by at least two independent methodologies and at five depth intervals. Pseudomonadota, Actinomycetota and Bacillota were described as the most common phyla identified in the continental deep subsurface and members of these representative genera had been previously identified in several other continental deep subsurface drilling operations. Using the genomic information from isolated microorganisms we could demonstrate their capacity to maintain the identified coupled biogeochemical cycles in operation. Complementary analytical techniques proved the presence in the deep subsurface of the different metabolic substrates and products of the identified biogeochemical cycles.
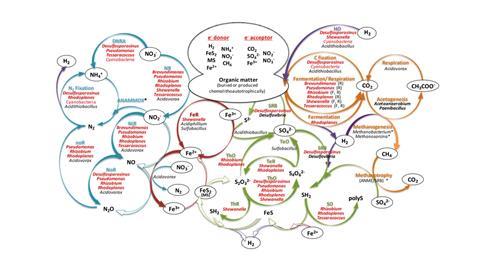
The activation of Fe oxidation in anaerobic conditions after the addition of water to dry core samples with high pyrite content, visualised by the formation of Fe3+ precipitates, strongly suggests the existence of anaerobic Fe oxidising activities in the subsurface of the IP. Fe oxidising activities pertain to a metabolic reaction of importance not only in this ecosystem but also in applied biotechnological processes such as biohydrometallurgy. Isolates from four of the most representative microorganisms identified in the borehole, belonging to the Acidovorax, Tessaracoccus, Pseudomonas and Shewanella genera showed efficient in vitro oxidation of ferrous iron using nitrate as electron acceptor for anaerobic respiration. The genomic analysis of these microorganisms showed the lack of recognisable genes associated with this metabolic activity, leading us to conclude that the observed oxidation of iron, at least for the tested microorganisms, is due to the chemical oxidation of Fe promoted by the high oxidising capacity of nitrite and nitric oxide generated by the use of nitrate as electron acceptor. These results strongly support the notion advanced by other authors that some nitrate reducers can efficiently generate reactive nitrogen species capable of oxidising iron under strict anaerobic conditions.
Remarkably, 82% of the most representative genera identified in this work have been described as putative nitrate reducers. In fact, we have identified the presence of genes coding for nitrate reduction in eight of the nine sequenced genomes isolated from the IPB subsurface. This result, together with the Fe2+ oxidation capacity shown by some IPB isolates, strongly suggests the importance of this metabolic activity in the IPB subsurface (the origin of the high concentration of secondary Fe minerals and the presence of soluble Fe along the column) as the source of the high concentration of iron detected in the Río Tinto basin.

From this work we can conclude that not only is there an important level of microbial biodiversity in the dark biosphere but that their metabolic activity can participate in the most important biogeochemical cycles. Obviously, more complementary information from devoted drillings in other mineral substrates is needed before we can thoroughly understand the role of the dark biosphere in the operation of planet Earth and other planetary bodies of our solar system and beyond.
The origin of this study was an Advance ERC project (grant 250/350- IPBSL, Iberian Pyrite Belt Subsurface Life Detection) granted to the Centro de Astrobiología to understand the origin of the extreme conditions detected in the Río Tinto, Iberian Pyrite Belt.








No comments yet