Not all elephant postmortems end in fire, but this one did. The team gathered mopane – ideal firewood as it burns very hot – from the bush, piled it on the carcass, and lit it.
Early investigations
This dramatic finale was in fact a safety precaution; burning a carcass is one way to prevent it from contaminating the environment with anthrax (Bacillus anthracis) spores if it’s infected. And Dr Chris Foggin, the wildlife vet who attended this postmortem, suspected that this elephant had died of anthrax.
Anthrax deaths are not uncommon here in northwestern Zimbabwe as the dry season heats up before the rains. It was August 2020 – a strange time in Victoria Falls. In the midst of the COVID-19 pandemic, intercity travel in Zimbabwe was banned and international travel restrictions had left the tourism hotspot a virtual ghost town. But over the next few weeks, Dr Foggin of Victoria Falls Wildlife Trust (VFWT), a local nonprofit organisation located about 10 km from the centre of town, would be busy with reports of dead elephants. The Trust operates under a Memorandum of Understanding with the Zimbabwe Parks and Wildlife Management Authority to support conservation activities in the Victoria Falls area. This includes a variety of fieldwork, including removing poachers’ snares from wildlife and conducting postmortem examinations as the need arises. Two more elephant carcasses were reported before the first had even been incinerated.
The next morning, Dr Foggin collected blood smears from the new carcasses but didn’t open them, assuming an outbreak of anthrax was now in full swing. He returned to the VFWT offices, which include a registered veterinary diagnostic lab, to examine the slides. When it comes to location, the VFWT Laboratory is hard to beat. It is nestled in the Victoria Falls National Park, surrounded by mixed mopane woodlands with free-ranging impala, bushbuck, kudu, buffalo, elephant, and the occasional giraffe, lion, or leopard. Just outside the laboratory is VFWT’s wildlife rehabilitation centre, which houses a variety of cases at any given time, plus permanent resident and ambassador bird Judge, a white-backed vulture.

Maintaining a laboratory in this part of the world comes with its own challenges. An inconsistent power supply makes a substantial backup generator essential. Many supplies and reagents must be imported, and having equipment serviced or calibrated is rarely straightforward. The Kalahari sand leaves a film of fine red dust everywhere. Elephants occasionally made mischief after hours – breaking off water taps, smashing rubbish bins, and once damaging the lab’s roof – before a fence was installed. Still, the laboratory is an invaluable resource to be able to diagnose some wildlife diseases on-site rather than sending samples off elsewhere. The blood smears from the elephants were stained with Giemsa, but surprisingly, none showed the characteristic magenta of anthrax bacilli (and PCR performed later was also negative for anthrax). Two smears did have some bipolar, short-rod, or coccobacilli bacteria.
Anthrax went on the list of ruled-out causes of death, along with starvation (the elephants were in reasonable body condition), poaching (the ivory was still intact), trauma (no external wounds), and poisoning (a test for cyanide, the most common poison in the area, was negative and no other species such as vultures were found dead). With the risk of anthrax eliminated, it now made sense to open the remaining carcasses. It was back to the field in a Landcruiser packed with kit akin to a horror film set: a box full of knives, an axe, saws, a cutting board, sample containers, a canvas bag full of thick ropes, a shovel, and more.
Postmortems, PCR and the case for P. multocida
Performing a complete postmortem examination of an elephant is a major undertaking. Opening the animal in the first place isn’t a quick job, as its tough skin dulls knives within a few strokes. Removing the massive organs is messy work, and the smell of decomposition and intestinal contents can be overpowering. Once the carcass is open, it’s usually not long before vultures begin circling in the sky above and perching in nearby trees, eagerly awaiting their turn at the carcass. The scavengers usually have a while to wait for their feast though, as the postmortem is a physically demanding job which can take hours. It is particularly draining in the hot dry season, when temperatures can exceed 38°C (100 F) and most postmortems are conducted in the full heat of the sun while wearing full-length coveralls, gloves, and heavy rubber boots.
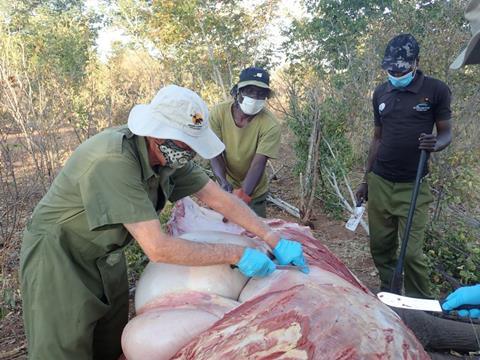
As Dr Foggin began his work, another report of a carcass came in. Only two of the three elephants could be sampled, given the late hour, but conducting two elephant postmortems makes for a very long day. There was no rest the next day, because another five carcasses were found scattered in the same general area, all appearing to have died around the same time as the previous elephants. Performing five full postmortems in different locations was too ambitious for one day, and the carcasses were decomposing in earnest now, so only limited samples could be taken. Organ samples were collected in formalin for histopathology at the VFWT Lab, one of the few in this region with on-site capacity.
What was evident from the carcasses that were examined in more detail was that there were haemorrhages across multiple organs, and usually an enlarged liver and spleen. At the lab, Dr Foggin examined histopathology slides of the formalin-fixed tissues and found acute inflammation in the liver, spleen, and lymph node, with Gram-negative coccobacilli colonies. Most of the blood smears contained paired coccobacilli as well. The puzzle pieces started to come together, pointing towards one thing: haemorrhagic septicaemia.
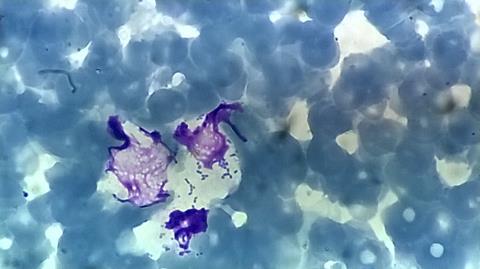
Haemorrhagic septicaemia is caused by Pasteurella multocida, a Gram-negative coccobacillus with multiple capsule types. The bacteria spread through the blood throughout the body, resulting in a pronounced inflammatory response. The disease is seen most often in cattle and water buffalo, with a rapid onset that is usually fatal. It’s been reported in Asian elephants, known to cause rapid death that looks similar to anthrax cases. P. multocida is an interesting bacterium: it is part of the normal respiratory flora of many mammals, but also capable of inducing a variety of serious diseases, including haemorrhagic septicaemia as well as fowl cholera in poultry. The highly publicised deaths of over 200,000 saiga antelope in Kazakhstan in 2015 were a stunning example of P. multocida at its deadliest. You may have even had a brush with it yourself - if you’ve ever had a cat bite that developed a nasty infection, it was likely thanks to P. multocida from the cat’s mouth.
African elephants, however, had not previously been reported to develop haemorrhagic septicaemia from P. multocida. Initial results from one sample submitted for culture confirmed P. multocida, and Dr Foggin and I began writing up a manuscript on this event as it was happening. Additional carcasses were reported over the next few weeks and teams went up in a helicopter and later a fixed-wing aircraft to search for more. A ground team went in to collect samples where it was possible; the carcasses found by the helicopter appeared to have died around the same time as the first cases. By early November, 35 elephants were thought to have died of haemorrhagic septicaemia.
For a thorough investigation, samples were submitted to other laboratories outside Zimbabwe for a variety of tests. Moving wildlife samples from one country to another is not as simple as sticking them in a box and sending them on their merry way at a Fedex office. First, import permits from veterinary and sometimes wildlife officials need to be requested from the destination country, and only once those are in hand can export permits be obtained from the veterinary department and CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) authority in the home country. These are the same permits you’d need to move a live wild animal or hunting trophies, and it can sometimes take weeks or months to receive them – less than ideal for bacterial culture samples. Once necessary permits were obtained, samples went off to laboratories in South Africa and the UK.
Further clues and suspected cause of death
Results trickled in. Other pathologists confirmed the bacterial septicaemia that Dr Foggin saw on the slides. Identifying the bacteria involved was less straightforward. Bacteria isolated from culture samples sent to Dr Marijke Henton in South Africa appeared to be P. multocida, as expected. But they didn’t react with any of the P. multocida capsular types, including the one most commonly associated with haemorrhagic septicaemia. The isolates were untypable on every one of five attempts made by Dr Angela Buys, and every time the controls had worked fine. Off more samples went for 16S rDNA sequencing, and finally came an unexpected answer: Bisgaard taxon 45.
I will admit to never having heard of this organism, and a literature review revealed rather little about Bisgaard taxon 45. Bisgaard taxa are unclassified members of the Pasteurellaceae family, named for Dr Magne Bisgaard, who has studied them extensively. Bisgaard taxon 45 is a P. multocida-like bacterium which has the unusual characteristic of being sucrose-negative (that is, it does not ferment sucrose like the classical P. multocida subsp. multocida). In short, it’s a close relative with a few key differences in how it responds to biochemical testing. The original strains that were grouped into Bisgaard taxon 45 were from lion and tiger bite wounds in humans, a chipmunk, and an unknown source. It had also been identified in healthy captive psittacines, an amazon parrot and a kea. It had not, however, been identified in elephants or in association with septicaemia.

Our investigation wasn’t over yet; with this new lead, the international team kept working to confirm Bisgaard taxon 45 and rule out other potential causes. Bisgaard taxon 45 was detected on FTA cards from more elephants – six in total – using 16S rDNA sequencing. Comparison of 16S sequencing samples in South Africa found they were identical to the sequence of a sample in the UK. Whole genome sequencing of the sample in the UK found that the genome clustered just outside P. multocida genomes, but not with other Pasteurella spp. Analysis of the genome confirmed encoding for 22 of the 25 Pasteurella virulence factors Dr Arnoud van Vliet tested for. One of the virulence factor genes present was pmHAS for hyaluronidase, associated with P. multocida type B isolates that cause haemorrhagic septicaemia. All in all, the results supported the presence of Bisgaard taxon 45 in multiple elephants, and the close genetic relationship and shared virulence factors of Bisgaard taxon 45 with P. multocida provided an explanation for the pathology observed.
Evidence from the past suggests that this wasn’t the first time that elephants died of septicaemia here. Dr Foggin reviewed records from his database and found dozens of cases of elephants that had died suddenly and were anthrax suspects that ultimately tested negative. In cases where anthrax was suspected and only a blood smear was collected, no further samples would have been available to test if anthrax was not present. Septicaemia could have been missed in those animals in the past. A case from an elephant calf, submitted a month prior to the 2020 mortality event, showed remarkably similar histopathology in the brain, liver, and spleen, with bacterial colonies present. Dr Foggin went back to slides from elephants that had died in nearby Hwange National Park in 2019, and once again found similar pathology in the liver and spleen. Bisgaard taxon 45 may have been causing sporadic septicaemia cases and simply gone undetected.
More results from samples sent to other labs in the UK, coordinated by Dr Falko Steinbach, helped to rule out other causes of death. Toxicology testing showed the samples did not contain cyanide, tetrodotoxin, or cyanobacterial toxins saxitoxin, anatoxin-a, or cylindrospermopsin, and none of the incidental toxicology findings could explain the septicaemia observed. No viruses could be detected from samples analysed by microarray or whole-genome sequencing. There were no compelling signs pointing in other directions away from Bisgaard taxon 45. The identification of Bisgaard taxon 45, rather than P. multocida, as the culprit meant I had to make major revisions to the original manuscript in progress. Over time, we made new additions with the many analyses that went into the study, ultimately resulting in a long and unwieldy draft. A major rewrite was clearly needed – again. Dr van Vliet and I worked to streamline the methods and results sections, relegating many details to the supplementary information, while completely overhauling the introduction and discussion sections into a much more concise product that was ultimately ready for submission to a journal. The acceptance at Nature Communications, more than seven months after submission, was a satisfying end to a multi-year effort for our interdisciplinary team.

Case closed?
There are still many questions that remain unanswered about Bisgaard taxon 45 and this mortality event. We don’t yet know how the bacteria came to be in this landscape – has it always been here? Is it typically found in elephants but hadn’t been previously recognised? The strain from the elephants was most closely related to one from a tiger bite wound in a man in the United States. There are no tigers here and none of the elephants examined had evidence of bite wounds from large carnivores, but if Bisgaard taxon 45 is in big cats here (lions or leopards), how did it infect elephants? Why did numerous elephants suddenly die in this relatively small area over this period in 2020, and will it happen again? We hypothesised that heat and drought may have added extra stress at a difficult time of year for elephants in a population with high competition for resources, but don’t know for sure. A single elephant was found in November, some distance from the earlier cases and weeks after the last carcass had been found – were other cases missed in the space and time between these deaths? VFWT has built culture and PCR capacity for Pasteurella at its lab and will continue looking for Bisgaard taxon 45 in elephants and large carnivores, slowly trying to piece together answers about this organism in this region.
The area where the mortalities occurred lies close to the heart of the Kavango-Zambezi (KAZA) Transfrontier Conservation Area, which is home to the largest remaining population of elephants in Africa. It encompasses about 520,000 km2 of land across Angola, Botswana, Namibia, Zambia, and Zimbabwe, with the vast majority of elephants concentrated in Botswana and Zimbabwe. Finding septicaemia as a cause of sudden mortality here gives wildlife veterinarians and conservationists a new and important differential diagnosis to consider going forward. Whether there is any population-level effect of septicaemia mortalities – or the potential for a large-scale mortality event like the saiga die-off – is unknown. We hope further study can improve our understanding of Bisgaard taxon 45 in the context of elephant conservation and reveal more about the role this organism plays in the KAZA landscape.










No comments yet