A new review highlights a pivotal shift from conventional luciferase and fluorescent protein systems toward self-sustaining luminescent platforms, particularly the fungal bioluminescence pathway (FBP).

By comparing strengths, limitations, and emerging innovations across technologies, the researchers argue that autonomous, substrate-free luminescence—especially when combined with artificial intelligence–driven protein design—could enable long-term, non-invasive monitoring in complex organisms.
Early tracing approaches relied heavily on radioactive or stable isotope labeling, which offered quantitative power but raised concerns over safety, cost, and limited real-time observation. Luminescence-based methods emerged as powerful alternatives. Luciferase reporters provide exceptional sensitivity by generating light through enzyme–substrate reactions, while fluorescent proteins allow high spatial resolution through external excitation.
READ MORE: New microscope harnesses bioluminescence to bring glowing cells into focus
READ MORE: New diagnostic tool uses bioluminescence to detect viruses
However, both approaches face intrinsic constraints: luciferases depend on repeated substrate delivery, and fluorescent proteins require excitation light that can cause phototoxicity, autofluorescence, and signal loss over time. These limitations have driven the search for next-generation systems that can operate autonomously within living cells and organisms, motivating renewed interest in naturally self-sustaining luminescent pathways and their potential redesign through modern synthetic biology and computational tools.
Paradigm shift
A study (DOI: 10.1016/j.bidere.2025.100060) published in BioDesign Research on 31 October 2025 by Hao Du’s team, Zhejiang University, reveals that self-sustaining luminescent systems, particularly the fungal bioluminescence pathway, represent a paradigm shift capable of overcoming substrate dependence and phototoxicity while enabling continuous, real-time biological tracing.
In outlining the main content, this review systematically compares three major luminescence platforms: luciferase-based bioluminescence, fluorescent protein–based imaging, and the fungal bioluminescence pathway. It first details how firefly and marine luciferases have become indispensable tools for gene expression analysis, protein–protein interaction assays, and in vivo imaging, while also emphasizing persistent drawbacks such as limited tissue penetration, signal variability, and the logistical burden of substrate administration.
The discussion then turns to fluorescent proteins, which revolutionized cell biology through multicolor labeling and high-resolution imaging but remain constrained by photobleaching, excitation-induced damage, and challenges in quantitative analysis, particularly in complex tissues such as plants.
Fungal bioluminescence pathway
The core of the review focuses on the fungal bioluminescence pathway, a four-enzyme metabolic cycle that converts endogenous metabolites into visible light without external substrates or excitation. Because its key precursor, caffeic acid, is widely present in plants and other organisms, the FBP can function autonomously when heterologously expressed.
The review summarizes successful demonstrations of glowing plants, mammalian cells, and other systems, highlighting advantages such as reduced toxicity, long-term signal stability, and suitability for high-throughput screening.
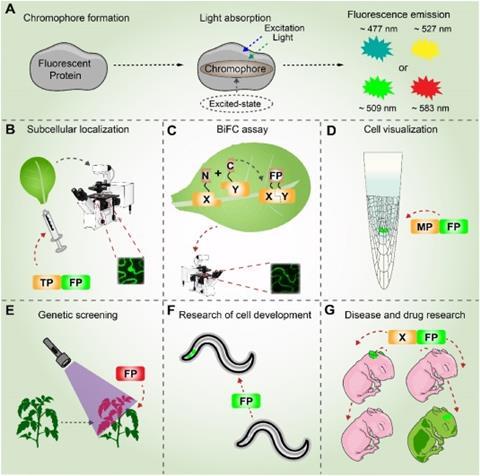
Importantly, the authors discuss how recent advances in enzyme engineering, metabolic optimization, and synthetic regulatory circuits are beginning to address current limitations in brightness, metabolic burden, and spectral diversity. The integration of artificial intelligence for de novo protein design and pathway optimization is presented as a critical strategy to further enhance performance and expand application scenarios.
Rapidly evolving field
In summary, this review positions luminescence-based tracing as a rapidly evolving field moving toward intelligent, autonomous systems. By synthesizing progress across luciferases, fluorescent proteins, and fungal bioluminescence, the researchers conclude that future breakthroughs will depend on combining protein engineering, AI-driven design, and metabolic control.
Ultimately, the work underscores a broader vision in which self-sustaining luminescent technologies become foundational tools for understanding and managing complex biological and ecological systems in real time.


No comments yet