Scientists who discovered 119-million-year-old selfish genes in yeast say the find potentially alters our understanding of how parasitic DNA impacts genome evolution.
Meiotic drivers, a type of selfish gene, are present in the genomes of nearly all species, including humans, where they unfairly transfer their genetic material to more than half of their offspring, sometimes leading to infertility, and decreased organism health.
Because of their parasitic potential, their longevity over evolutionary time is believed to be short-lived, until now.
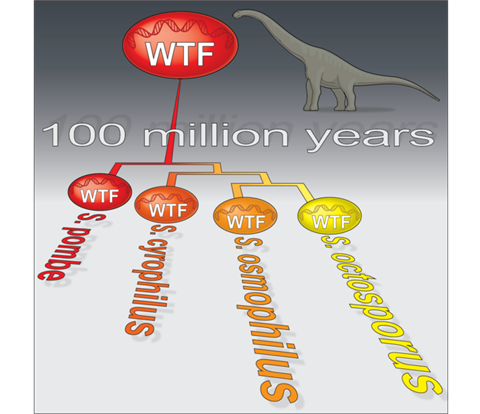
New research from the Stowers Institute for Medical Research, in collaboration with the National Institute for Biological Sciences in Beijing, China, has uncovered a selfish gene family that has survived for over 100 million years—10 times longer than any meiotic driver ever identified—casting new doubt on established beliefs on how natural selection and evolution tackle these threatening sequences.
“The thinking has always been that because these genes are so nasty, they won’t stick around in populations for very long,” said Associate Investigator SaraH Zanders, PhD.
“We just found out that it isn’t true, that the genomes simply can’t always get rid of them.”
Meiotic drivers are thus named as they can acquire the ability to literally “drive” the transmission of their genes throughout a genome, often with negative consequences. Natural selection is therefore the major force counteracting selfish genes, favoring genetic variants that kill drive for the recovery of fertility and overall health of a species.
“Natural selection has a limited ability to remove meiotic drivers from a population,” said Zanders.
“Imagine holding soccer team tryouts (natural selection) to recruit the best players (genes that promote fitness). Drivers are players that sabotage the other players trying out. Drivers make the team, but not because they are good at soccer.”
In a recent study published in eLife on October 13, 2022, led by researcher Mickael De Carvalho, PhD, from the Zanders Lab, and Guo-Song Jia, a predoctoral researcher in the lab of Li-Lin Du, PhD, identified for the first time that a family of selfish genes called wtf have not only flourished in the fission yeast, Schizosaccharomyces pombe, but have been passed on to three unique yeast species that diverged from S. pombe around 119 million years ago.
“This finding is particularly novel as a family of drive genes have thrived at least ten times longer than what geneticists ever believed possible,” said Zanders.
During meiosis, the specialized cell division that gives rise to reproductive cells like sperm and eggs, the inheritance of genetic material from a set of chromosomes from each parent is 50/50, or equally probable for each reproductive cell.
Meiotic drivers in yeast are in fact a more potent genetic parasite. The wtf gene family are killer meiotic drivers; they not only transmit the selfish gene to over 50 percent of offspring but then destroy the reproductive cells—or spores in yeast—that do not inherit the drive gene.
Natural selection in a genome typically rescues a species from selfish genes by favoring genes that suppress, or silence drive, rendering it useless. How the wtf gene family evaded suppression is largely due to their rapid rates of mutation.
This persistence alters our perception of how a species can overcome the expected increase in infertility that typically leads to extinction. It also changes the way scientists may look for and identify families of selfish genes in different species, including humans.
“Until now, when looking for candidate drivers within a genome, I wouldn’t have considered “old” genes as a possibility,” said Zanders. “Since selfish genes are major drivers of evolution, this new finding opens the door for thinking about how drivers can have persistent, long-term effects on genome evolution.”
Additional authors include Ananya Nidamangala Srinivasa, R Blake Billmyre, PhD, Jeffery J Lange, PhD, and Ibrahim M Sabbarini.
This work was funded by the New Innovator Award of the National Institutes for Health (award: DP2GM132936), institutional support from the Stowers Institute for Medical Research, the Chinese Ministry of Science and Technology, and the Beijing Municipal Government.







No comments yet