Custom-built extracellular membrane vesicles (MVs) can be deployed as a microbe-free way of boosting crop growth without the downsides of plant-growth promoting rhizobacteria (PGPR), a new study reveals.

The research by a team at the University of Seville proposes that if a protein of interest is fused to extracellular membrane vesicle scaffolding proteins, it will be shuttled into the extracellular membrane vesicle cargo, which can be delivered intra- and interspecies as well as interkingdom.
The study is outlined in a paper, ‘Membrane vesicle engineering with “à la carte” bacterial-immunogenic molecules for organism-free plant vaccination’ which has been accepted by Microbial Biotechnology, an Applied Microbiology International publication.
Sustainable agriculture
Corresponding author Dr Jose Borrero de Acuña explained: “In recent years, PGPRs have emerged as a crucial component of sustainable agriculture, promoting the prophylaxis and therapy of crop-associated soils. They play a vital role in ensuring agricultural sustainability by substituting agrochemicals and enhancing primary food production.
“While some PGPRs have already been successfully commercialised to improve crop yields, the persistence of beneficial microbes as inoculants for important food crops is shadowed by lower persistence in soils and suboptimal rhizosphere colonisation abilities, particularly when competing with better-adapted indigenous microbes.
“Moreover, undesirable down-regulation of plant-growth promotion traits to conserve energy and resources, as well as promiscuous host-specificity that can enhance the growth of wild or invasive plant species, pose challenges.
“In our article, we discuss the potential solution of using tailored extracellular MVs as microorganism-free bioagents to address the ecological and biological limitations of natural PGPR in agriculture.”
Meeting of minds
Drs. Francisco Pérez Montaño, Francisco Javier López Baena and Irene Jiménez Guerrero’s research group at the University of Seville have been studying the molecular bases governing plant-microorganism interactions for more than 25 years to develop new strategies for plant growth promotion and phytopathogen biocontrol. In 2021, Dr. Borrero de Acuña, previously located at the Technical University Braunschweig, Germany, joined the Department of Microbiology, University of Seville.
Working in cooperation with Dr. Dieter Jahn (Technical University Braunschweig, Germany) and Dr. Martin Warren (University of Kent, England), he has been focused on clarifying the underlying mechanisms for extracellular membrane vesicle formation - nanoparticles which are lumen-containing spheres of lipidic nature released to the extracellular environment by the three domains of life.
Importantly, they are investigating a number of scaffolding proteins that orchestrate extracellular membrane vesicle formation in bacteria.
The convergence of these areas of expertise led to the idea of loading extracellular membrane vesicles with proteins that induce a defensive response in plants, ultimately aiming to develop molecular vaccines for agriculturally relevant crops, says corresponding author Dr. Borrero de Acuña.
Microbe-free bioagents
“We are exploring the utilisation of customised extracellular membrane vesicles (MVs) as microorganism-free bioagents to address the ecological and biological limitations of natural plant growth promoting rhizobacteria (PGPR) in agriculture,” he says.
“In this context, we emphasise MV engineering to encapsulate immunomodulatory effectors within their cargo for use as biocontrol agents for sustainable agriculture strategies. The ultimate objective is to induce plant priming by harnessing its innate immune responses, thereby preemptively preventing future infections.
“The overarching aim is to avoid crop infections that represent a significant burden to the economic system due to crop losses and endanger the constant supply of food to an ever-increasing population.”
Scaffolding proteins
The paper described a group of conserved proteins that are involved in active membrane vesicle formation or fundamental building blocks of its architecture.
“The fusion of such extracellular membrane vesicle scaffolding proteins to a protein of interest resulted in its shuttling into the extracellular membrane vesicle cargo,” Dr Borrero de Acuña said.
“These vesicles, as described in the literature, can deliver their cargo intra- interspecies and interkingdom - hence, the applications are boundless.”
Tried and tested strategies
The team envision two previously tested strategies for MV engineering in Gram-negative bacteria:
i) creating chimeric proteins by fusing the MV scaffold protein with the protein of interest, ensuring co-transportation into the MVs
ii) generating recombinant proteins fused with a signal peptide for translocation into the periplasmic space and subsequent packaging into the MVs.
“These technologies present the opportunity to purify naturally produced MVs loaded with immunogenic molecules from plant pathogens. Consequently, engineered MVs can serve as a platform for developing organism-free plant vaccines,” Dr Pérez-Montaño said.
A la carte engineering
One aspect that proved surprising was the versatility of the system given that virtually any protein of interest can be fused to such scaffolding proteins and shuttled into the MV cargo.
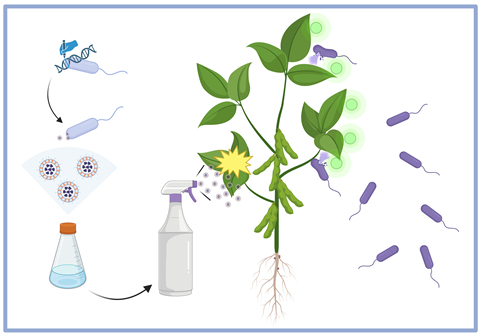
“This opens unlimited possibilities of ‘à la carte’ membrane vesicle engineering that brings not only to the field of sustainable agriculture but also to biomedicine,” Dr Borrero de Acuña said.
The next step will now be to obtain MVs in large quantities by scaling up the generation and isolation process, and to test the hypothesis not only under controlled conditions but in the field.
This work was funded with grants from the “Ministerio de Ciencia e Innovación” of the Spanish government and the “Consejería de Universidad, Investigación e Innovación” of the Andalucian government.
‘Membrane vesicle engineering with “à la carte” bacterial-immunogenic molecules for organism-free plant vaccination’ which has been accepted by Microbial Biotechnology, an Applied Microbiology International publication.
Topics
- Applied Microbiology International
- Bacteria
- biomedicine
- Borrero de Acuña
- Community
- Dieter Jahn
- extracellular membrane vesicles
- Francisco Javier López Baena
- Francisco Pérez Montaño
- Healthy Land
- Irene Jiménez Guerrero
- Jose Borrero de Acuña
- Martin Warren
- New nitrogen-fixing bacteria for sustainable agriculture
- plant-growth promoting rhizobacteria
- Research News
- Soil & Plant Science
- Technical University Braunschweig
- UK & Rest of Europe
- University of Seville





No comments yet