A simple new method of disinfecting drinking water is based on tiny biocompatible assemblies of atoms, known as quantum dots, made of silver sulphide with caps made of a silver-binding peptide.

When irradiated with near-infrared light, they kill bacteria in water with high efficiency through synergistic effects.
The new method is described by a Chinese research team in the journal Angewandte Chemie.
In some areas of the world, it can be very difficult to access clean drinking water. Pathogenic bacteria such as E. coli, enterococci, salmonella, or cholera pathogens can cause serious infections.
Traditional disinfection methods widely implemented in recent decades, such as UV light, chlorination, and ozone, have disadvantages, including high costs, poor efficiency, poor biocompatibility, and carcinogenic by-products.
A team led by Xushen Qiu, Wei Wei, and Jing Zhao has introduced an alternative new method that is based on quantum dots made of silver sulphide (Ag2S). Quantum dots are nanoscopic structures made of about one-to-ten thousand atoms that are “confined” in space. Their quantum-mechanical properties correspond more to those of molecules than macroscopic solids, which can lead to interesting opto-electronic effects.
Medical use
Silver sulphide quantum dots are already used in photodynamic and photothermic therapy, including for the treatment of certain tumours and skin diseases. They can be used as contrast agents and as fluorescence thermometers.
So far, they have not been used much for disinfecting water, partly because previous methods for preparing them have been complicated and expensive. The team from Nanjing University and the Nanchuang (Jiangsu) Institute of Chemistry and Health has now developed a simple, inexpensive production method, in which the quantum dots are enclosed by caps made from a specially developed biomimetic silver-binding peptide (AgBP2).
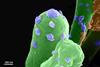
When irradiated with near-infrared (NIR) light, the new AgBP2-Ag2S quantum dots effectively kill bacteria in water. They are chemically stable, photostable, and biocompatible.
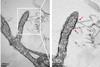
Their strong activity is due to a synergistic combination of two effects. First, irradiation causes them to produce highly reactive oxygen species, and second, they cause strong local heating. Neither of the two effects alone leads to success, but their synergistic combination effectively destroys bacterial cell membranes. They are able to kill over 99% of E. coli bacteria within 25 minutes of NIR irradiation—a highly promising strategy for antibacterial disinfection of water.





No comments yet