A ruling overturning the Affordable Care Act (ACA) coverage mandate has the potential to dramatically change the landscape for early detection and treatment of hepatitis C virus in the U.S., according to a new paper published in Gastro Hep Advances.
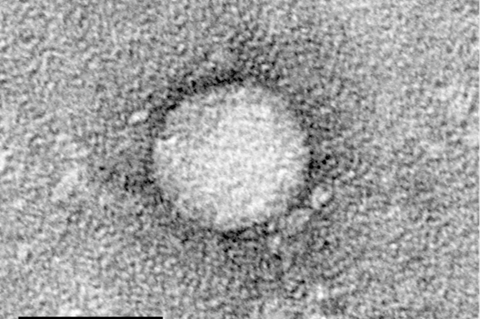
Kennedy v. Braidwood (formerly, Braidwood v. Becerra), is a United States Supreme Court case challenging the ACA requirement that insurance plans cover preventive services receiving an A or B rating by the United States Preventive Services Task Force (USPSTF) at no cost to patients. Since screening for HCV is among these services, a ruling overturning the ACA coverage mandate would reverse progress in HCV screening and treatment, leading to more undiagnosed infections, preventable cancers, and deaths.
READ MORE: New hope in the fight against Hepatitis C: Broadly effective innovative vaccine design
READ MORE: Hepatitis C leaves ‘scars’ in immune cells even after successful treatment
More than 2 million Americans are currently living with hepatitis C, despite the availability of a highly accurate screening tests and curative treatments. Alarmingly, HCV prevalence has more than doubled over the past decade and is projected to continue to rise.
HCV is curable, yet screening rates are low, especially among low-income populations, where people are five times more likely to have the virus.
Direct-acting antivirals
Treatment with direct-acting antivirals (DAAs) leads to a cure in virtually all cases. Without treatment, patients are at high risk of developing cirrhosis and hepatocellular carcinoma.
After the USPSTF endorsed one-time HCV screening for all adults aged ≥18 years old in 2020, eliminating out-of-pocket costs for patients under the ACA, screening rates rose from 141 to 253 per 1,000 person-years among pregnant women and from 29 to 37 per 1,000 person-years among non-pregnant women. As a result of higher detection, in 2022 the rate of reported acute HCV infections decreased for the first time in more than a decade.
More information about hepatitis C is available in the AGA GI Patient Center.
AGA is also deeply concerned about the impact of Kennedy v. Braidwood on colorectal cancer screening and prevention. In 2025, over 150,000 people will be diagnosed with CRC and over 50,000 people will die from the disease.
”If we take a step backward and patients must pay for screening, we can expect to see a 5.1% increase in CRC incidence and a 9.1% increase in CRC mortality,” the authors said.






No comments yet