Targeting malignant tumours with high precision is challenging for biomedical researchers. However, this scenario is likely to witness a paradigm shift in the near future, through the use of specially engineered bacteria that can eliminate malignant cells efficiently.

Using bacteria to target cancer cells, or bacterial therapy, can be further enhanced through genetic engineering and nanotechnology. However, its efficacy may be hindered due to technical constraints and the potential development of antibiotic resistance. Hence, it is crucial to achieve the moderate yet effective chemical modification of bacteria for improved biocompatibility and functionality, such that their medical abilities are not compromised.
Recently, certain types of purple photosynthetic bacteria (PPSB) have come into the limelight for their potential to address the challenges of bacterial therapy. Astudy published in Nano Today reports the use of chemically modified PPSB for detecting and eliminating hard-to-eradicate cancerous cells in a mouse model.
Ideal bacterium
The study, led by Associate Professor Eijiro Miyako from the Japan Advanced Institute of Science and Technology (JAIST), selected Rhodopseudomonas palustris (RP) as the optimal bacterium for conducting the studies.
“RP demonstrated excellent properties, such as near-infrared (NIR) fluorescence, photothermal conversion, and low cytotoxicity. It absorbs NIR light and produces free radicals - a property that can be utilized to kill cancer cells,“ explains Prof. Miyako.
In an attempt to improve the therapeutic efficacy of the isolated strain, the team sought chemical modifications to alter the bacterial membranes. First, they performed membrane PEGylation, or the attachment of polyethylene glycol derivatives to the bacterial cell walls. Prior research indicates that bacterial PEGylation helps in evading host immune response and converts light energy into heat, which can then be utilized to selectively eliminate cancerous cells.
Encouraging results
The initial results were encouraging. For instance, coating the RP membrane surface with a “Biocompatible Anchor for Membrane (BAM)” did not adversely affect RP cell viability for at least a week. Moreover, the BAM-functionalized RPs were not eliminated via phagocytosis by macrophages - cells that play a key role in the immune system’s defensive actions against bacterial invasions.
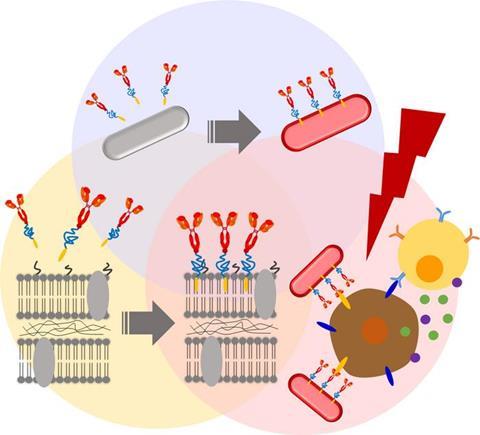
Next, the researchers attached a fluorescent “Alexa488-BSA” conjugate to the BAM-functionalized RPs, thus creating a bacterial complex with a trackable fluorescent marker. This conjugate was subsequently replaced with a “PD-L1” antibody.
Prior studies have shown that cancer cells express a protein called “Programmed Cell Death Ligand 1 (PD-L1)” on their surface. PD-L1 can smoothly turn off the host defense system by binding to PD-1 receptors. This allows the cancer cells to evade immune detection and elimination. Anti-PD-L1 antibodies block this interaction, thus preventing cancer cells from bypassing immune-system-mediated destruction.
Dramatic anticancer effect
As expected, both anti-PD-L1–BAM–RP and RP, inhibited tumour growth in a murine model of colon cancer. However, anti-PD-L1–BAM–RP, BAM–RP, and RP, when excited with a laser, showed an especially dramatic anticancer effect.
In fact, solid tumours vanished completely following the laser irradiation of anti-PD-L1–BAM–RP, BAM–RP, or RP that were injected into tumour-bearing mice. Further, on assessing photothermal conversion properties, both anti-PD-L1–BAM–RP and natural RP exhibited strong photothermal conversion due to the presence of light-driven bacteriochlorophyll (BChl) molecules.
Among the various bioconjugates, anti-PD-L1–BAM–RP showed the highest efficacy in the initial stage of the treatment. Moreover, it was not toxic to surrounding healthy cells or to the murine host. Subsequent experiments revealed the underlying mechanism of colon tumour annihilation in the mouse model.
Clinical trials in 10 years
“Our findings revealed that light-driven functional bacteria demonstrated effective optical and immunological functions in the murine model of colon cancer. Moreover, the NIR fluorescence of the engineered bacterial complexes was used to locate tumours, effectively paving the way for future clinical translation,” says Prof. Miyako.
He further adds, “We believe that this bacterial technology could be available for clinical trials in 10 years and have positive implications for cancer diagnosis and therapy.”



No comments yet