A new study has shown how oncoprotein CagA from Helicobacter pylori disrupts Wnt/PCP signalling and promotes gastric carcinogenesis.
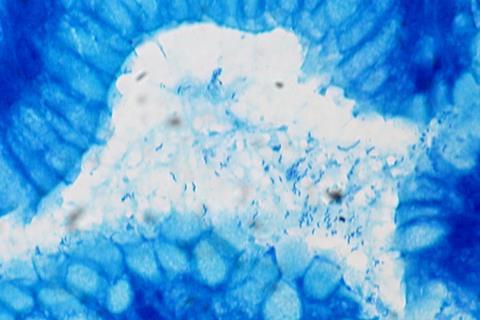
Helicobacter pylori (H. pylori) infections are commonly associated with abdominal pain, bloating, and acidity. Clinical evidence suggests that infection with H. pylori cagA+ strains dramatically increases the risk of developing gastric cancer.
A specialized protein delivered by H. pylori to the host, oncoprotein “CagA,” has been shown to interact with multiple host proteins and promote gastric carcinogenesis (transformation of normal cells to cancer cells). However, the underlying mechanisms associated with its biochemical activity have not been fully determined yet.
Uncovering pathways
A new study published in Science Signaling shares insights into the additional mechanism of oncogenic CagA action.
“CagA interacts with multiple host proteins within the gastric epithelial cells, thereby inducing pathways associated with oncogenesis and promoting gastric carcinogenesis. We were curious to find out which pathways were involved in this process,’ says Dr. Atsushi Takahashi-Kanemitsu, lead author of the study and Assistant Professor, Department of Biochemistry & Systems Biomedicine, Juntendo University, as he states the motivation behind pursuing this study.
In order to carry out their study, the researchers expressed oncoprotein CagA in three different models - embryos of Xenopus laevis (lab frog), adult mouse stomach, and cultured human gastric epithelial cells - and tried to understand its effect on the host cells and pathways.
Impairment of cell movements
The team noted that the expression of the CagA oncoprotein in X. laevis embryos led to impairment of convergent extension movements—cell movements observed during embryonic development that are involved in shaping or elongating organismal tissues and organs. This impairment further interfered with subsequent key embryonic development processes, including body axis formation.
Similarly, the team performed an experiment using adult mice. They generated genetically modified (transgenic) mice that specifically express the CagA oncoprotein in the stomach epithelial cells in response to tamoxifen treatment.
The researchers observed that CagA expression in the stomach of adult mice caused an increase in the depth of pyloric glands—secretory glands that facilitate digestion/stomach function—and also triggered abnormal/excessive cell multiplication, which is a phenomenon remarkably observed in various types of cancers.
This also led to the displacement of the proteins “VANGL1/2” - members of the Van Gogh-like (VANGL) protein family, which play key roles in various biological processes - from the plasma membrane to the cytoplasm. CagA expression also resulted in fewer differentiated enteroendocrine cells, which are specialized cells in the gastrointestinal tract that aid in digestion.
Human cells
Finally, the team expressed the CagA oncoprotein in cultured human gastric epithelial cells. The experiments clearly demonstrated that a small region of the CagA oncoprotein was interacting with amino acid residues from the proteins VANGL1/2, thus leading to its displacement (a phenomenon also observed in the mouse model) and resulting in disruption of the Wnt/PCP pathway—a key biological ‘relay’ that affects organismal development.
Corresponding author Masanori Hatakeyama, Laboratory Head, Institute of Microbial Chemistry, Microbial Chemistry Research Foundation, says: “Perturbation of Wnt/PCP signaling by the H. pylori CagA-VANGL interaction induces hyperplastic changes, along with impaired cell differentiation in gastric pyloric glands. This, in conjunction with other oncogenic CagA actions, may contribute to the development of gastric cancer.”
In summary, the researchers conclude that through this study, they were able to elucidate the molecular mechanisms involved in gastric carcinogenesis induced by H. pylori, gain insights into the role of the Wnt/PCP pathway in carcinogenesis, and propose it as a potential target for clinical interventions against H. pylori cagA+ infections.





No comments yet