Leishmaniasis, a disease that affects between 700,000 and 1 million people worldwide annually, is particularly prevalent in disadvantaged populations. Transmitted by parasites of the genus Leishmania through the bite of the Phlebotomus sandfly, cutaneous and visceral forms of the disease are endemic in Europe, with 80% of skin cases concentrated in the Eastern Mediterranean.
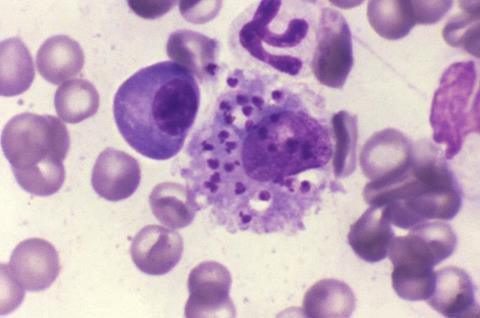
Current drugs to treat this disease face challenges such as resistance, toxicity, and high costs. Therefore, there is an urgent need to identify new therapeutic agents with innovative approaches to address these difficulties.
In this context, a team led by IBEC’s Nanomalaria research group and the Barcelona Institute for Global Health (ISGlobal) has explored an innovative approach to treating leishmaniasis based on recent findings in malaria.
Potent antimalarial drug
The research, published in the scientific journal Antimicrobial Agents and Chemotherapy, focuses on the in vitro action of the compound YAT2150, originally designed as a potent antimalarial drug. “As part of a previous project focused on improving the efficacy of known drugs against leishmaniasis through encapsulation, the idea arose to test the compound YAT2150. Surprisingly, it turned out to be very effective against leishmaniasis as well, putting us in a unique position, since both diseases are the main causes of parasite-induced mortality in humans,” explains Xavier Fernàndez Busquets, leader of the study and principal investigator at IBEC-ISGlobal.
The results of the study suggest that YAT2150 could act by inhibiting protein aggregation in Leishmania cells, the same mode of action that has already been observed in the malaria parasite. This ability to act effectively against both Plasmodium and Leishmania positions YAT2150 as a promising candidate for the treatment of co-infections of both parasites.
The team is currently working to move on to the next phase of research, which involves testing the drug in mouse models of leishmaniasis — a crucial step towards the development of an innovative treatment.
Power of encapsulation
The work also addresses the effects of encapsulating the drug within liposomes. This strategy not only expands its therapeutic window, enhancing its lethality against the parasite and reducing toxicity to the rest of the cells but also allows the drug to be directed specifically to the areas of the body of interest.
On the other hand, a key indicator to know the specificity of action of a drug is the selectivity index: “For a drug to be considered to reach development phases and clinical trials, its selectivity index must be greater than 100. In our case, we have found some drugs derived from YAT2150 that show selectivity rates for the malaria parasite of 1,000, 2,000, or even 3,000. These are very promising results,” says Lucía Román-Álamo, a PhD student in the Nanomalaria group and the first author of the study.
The work has also had the collaboration of Diego Muñoz-Torrero’s research group at the Institute of Biomedicine of the University of Barcelona (IBUB), which was responsible for the development of the family of drugs derived from the mother molecule YAT2150.
Topics
- Barcelona Institute for Global Health
- Diego Muñoz-Torrero
- Disease Treatment & Prevention
- Institute for Bioengineering of Catalonia
- leishmaniasis
- Lucía Román-Álamo
- One Health
- Pharmaceutical Microbiology
- Research News
- UK & Rest of Europe
- University of Barcelona
- Veterinary Medicine & Zoonoses
- Xavier Fernàndez Busquets





No comments yet