An experimental mRNA vaccine provides protection in preclinical animal models against infection from Borrelia burgdorferi, the bacterium that causes Lyme disease, according to new research from the Perelman School of Medicine at the University of Pennsylvania.
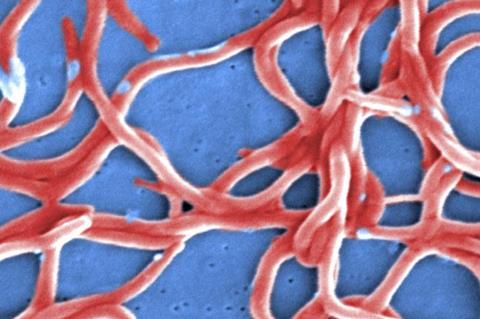
Results from these preclinical animal models suggest that the vaccine prevents the development of Lyme disease and may represent a powerful tool in reducing the number of Lyme disease cases.
The bacteria that causes Lyme disease is transmitted to humans through the bite of infected ticks, and can cause fever, headache, fatigue, and a skin rash. If left untreated, infection can spread to joints, the heart, and the nervous system.
Most cases of Lyme disease can be treated successfully with a few weeks of antibiotics, but some individuals develop post-treatment Lyme disease syndrome (PTLDS), which can cause long-lasting symptoms like severe joint pain and neurocognitive issues. While there are existing vaccines against Lyme disease for dogs, there is not currently one approved for routine use in humans.
Complex organisms
“Bacteria are more complex organisms than viruses, and therefore it can be more challenging to develop effective vaccines against them,” said senior author, Norbert Pardi, PhD, an assistant professor of Microbiology. “Here we were able to identify a target for a mRNA vaccine that shows promising results for preventing B. burgdorferi infection in animal models.”
The vaccine, described recently in Cell Press, uses the same messenger ribonucleic acid (mRNA) technology employed in the Pfizer and Moderna SARS-CoV-2 vaccines, which was pioneered at Penn.
Along with mRNA vaccine pioneer Drew Weissman, MD, PhD, the Roberts Family Professor in Vaccine Research and director of Vaccine Research at Penn Medicine, Pardi and his laboratory identified one of the proteins in B. burgdorferi that elicit a potent immune response, called outer surface protein A (OspA). OspA is a conserved protein in the multiple strains of B. burgdorferi, making it an ideal target for preventing an initial B. burgdorferi infection from progressing to Lyme disease.
Strong response
Tests in animal models showed that the mRNA vaccine targeting OspA induced a strong antigen-specific antibody and T-cell response after a single vaccination that could protect from infection of B. burgdorferi. What’s more, the vaccine elicited a strong memory B cell response, which can be activated much later to help prevent infection by B. burgdorferi long after the vaccine is administered.
“Cases of Lyme disease have been rising sharply in the United States, underscoring the need for a vaccine to protect individuals from infection,” said Pardi. “The mRNA technology shows great promise for use in developing a vaccine that may prevent Lyme disease and subsequent development of the debilitating symptoms of PTLDS.”



No comments yet