The John Innes Centre (JIC) is probably most famous for John Innes compost, but a lot more goes on there.
Situated on the Norwich Research Park, which contains the University of East Anglia (UEA), the JIC is known worldwide for the work it produces on plants and agriculture, including the plant microbiome and the infamous Streptomyces chemical factories, from which many modern drugs were developed. But how did the JIC become a world leader in its field? (Pun intended).

A week before he died in 1904, philanthropist John Innes wrote a will stating that the bulk of his fortune should be used to establish a horticultural school. This led to the John Innes Horticultural Institution being founded in Merton Park, London in 1910. Designed to be an advanced school for fruit breeding, it was headed by William Bateson, who had coined the word ‘genetics’ in 1905, and it was the UK’s first research centre for plant breeding. Early work focused on just that, with genetic studies performed on Pisum sativum (the pea that Mendel used in his infamous experiments, still studied at the JIC today) and breeding new varieties of plants for agriculture. Released in the 1930s onwards, several of these fruits can be found in supermarkets today. Summer Sun cherries, for example, which are known to produce large crops and are resistant to the spring frosts common in the UK. Researchers at the institution also developed the aphid-resistant Malling-Merton rootstock, still used widely in apple orchards. When growing apples and pears, it is rare to grow the tree from seed in place; instead, a faster-growing rootstock is planted and cut once the trunk is thick enough to support the fruit tree, which is then grafted on top of it. This means the fruit tree jumps straight to the crop-producing stage of its life, instead of taking years before producing enough apples to be commercially useful.
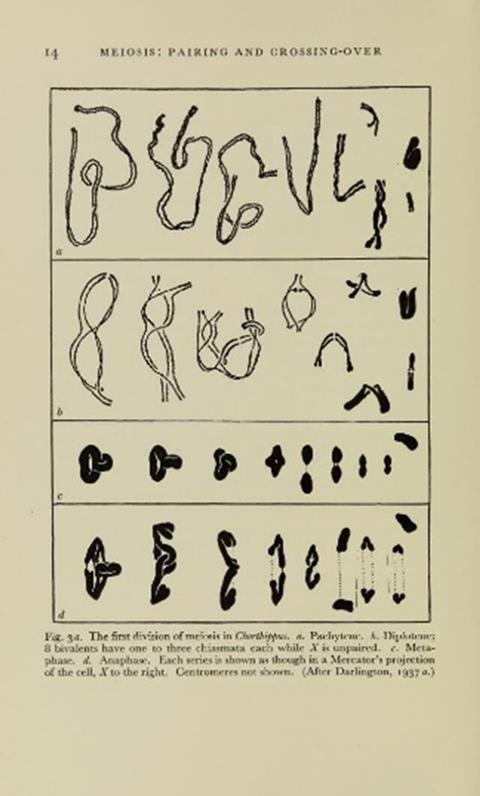
It wasn’t just plants studied at the JIC though. In 1937, J. Haldane, Head of Genetical Work at the institution together with Julia Bell at University College London jointly published the first evidence of sex-linkage in humans, showing that haemophilia and colour blindness were genetic diseases, now used as examples of such diseases in high schools. Not long after, in 1939, the then John Innes Director Cyril Darlington published a book ‘Evolution of Genetic Systems.’ This highly controversial piece introduced mitosis and meiosis to the world, discussing how chromosomes pair and recombine during meiosis and chromosomal crossing-over and how this related to heritability and genetics, explaining the genetic components of the diseases Haldane was working on at the time. Plenty of other genetics work was also on-going at this time, including the beginnings of modern genetic modification of crops and plant-associated bacteria. One of the most important of these discoveries was the Holliday junction in the yeast Saccharomyces cerevisiae, in 1964. Discovered by Robin Holliday, the Holliday junction is the four-way joining of DNA during double-strand break repair. A critical intermediate in homologous recombination, the Holliday junction is critical both to life and to genetic modification techniques, where it is still the foundation of modern genetic recombination modelling.
Although microbiology had always featured in the institution’s history, it was in 1968 that the JIC truly dived into microbiology by launching its Streptomyces genetics programme. The year before, the JIC had moved to its current location in the Norwich Research Park, where the grandfather of modern Streptomyces research, David Hopwood, was a professor at UEA. Hopwood’s group lay the groundwork for actinomycete research, mapping Streptomyces coelicolor and providing evidence for plasmid-determined mating, used today in genetic modification tools for actinomycetes. This work established S. coelicolor as the model organism for the genus, as well as placing the JIC and UEA at the vanguard of Streptomyces research. Only a couple of years later, genetic modification of Streptomyces using protoplasts was performed by members of Hopwood’s group and is since widely used by pharmaceutical companies in the search for novel antimicrobials. Protoplast genetic modification uses cells, normally algae, which have had their cell wall removed to create the titular protoplast. This allows for efficient transformation of plasmids from the protoplast into the target cell with high frequency. Given how difficult it is to modify the genomes of actinomycetes, this was a major breakthrough and is still used today in strains that have proven difficult to modify with other techniques.

The ability to manipulate the genome of actinomycetes led to the first ‘hybrid’ antibiotics to be produced at the JIC in 1985. Francisco Malpartida isolated the gene cluster responsible for the production of three different antibiotics in various Streptomyces strains and transferred them to alternative strains that produced antibiotics from the same chemical class. This led to the novel antimicrobials being produced as the biochemical machinery for one antibiotic was applied to the other, leading to major changes to the compound. This technique was used by pharmaceutical companies until recently to iterate on existing antibiotics, at least until funding for antibiotic research was cut by the major companies.
Today, the JIC is still at the forefront of research into plants, plant pathogens and the plant microbiome. In 2000, a team headed by Anne Osbourn discovered that oat plants produce antimicrobials in their root systems in an attempt to manipulate the root microbiome. This work, along with the work of others, has led onto the idea of ‘biocontrol’ in farming, inoculating the soil around crops with beneficial fungi and bacteria to defend crops from pathogens and increase yields, akin to probiotics in humans. Earlier this year (2019) Anne’s team worked jointly with the Chinese Academy of Sciences to show that even small changes in the chemicals produced by the model plant Arabidopsis thaliana caused major changes in the root microbiome. Research is now ongoing into how we can manipulate the production of these chemicals to alter the bacteria associated with crops to increase yield or disease resistance.

Back in 2002, researchers at the JIC also produced the first full-genome sequence of S. coelicolor with the help from the Sanger Centre, Cambridge. Understanding the whole genomes of actinomycetes has led to ‘genome mining’ of strains today, using pleotropic and genetic techniques to activate dormant biosynthetic gene clusters. Researchers in Matt Hutchings’ lab in UEA and Barrie Wilkinson’s in the JIC jointly discovered the novel antimicrobial formicamycin from an ant-associated Streptomyces strain by using CRISPR/cas9 to remove sections of the genome responsible for producing previously known antimicrobials to prevent the possibility of rediscovery. These same groups also work on understanding how Streptomyces and chemicals produced by plants interact to unlock these dormant gene clusters, both for use in agriculture and to fight new tools to fight antimicrobial resistance.

As science becomes ever more interdisciplinary, a large proportion of the work at the JIC focuses on bacteria, fungi and viruses, as human and plant pathogens and as sustainable replacements to agrichemicals. Genetic modification of plants to form new, better crops continues but so does research in understanding and combating the pathogens threatening food production. As climate change continues, reducing arable land and water availability, understanding how we can improve crop yields without using vast quantities of fertilisers and herbicides could help ensure we have enough food for everyone. Equally, the genetic tools discovered at the JIC, and used around the world today, are finding new antimicrobials to protect us from the other major threat to modern civilisation – antimicrobial resistance. By placing itself at the forefront of such critical issues, the JIC, UEA and the entire research park in Norwich is sure to be busy for a long time into the futu






No comments yet