Antimicrobial resistance (AMR) is a global health challenge, undermining the efficacy of antimicrobial treatments for infectious diseases. Novel antimicrobials are desperately needed to combat the AMR crisis, however, the clinical and preclinical pipeline for novel antimicrobials is virtually stagnant, with only 13 novel antibiotics being approved for use in humans in the last decade. Only 2 of these 13 antibiotics are truly novel chemical entities, with the others representing modifications of existing drug classes. Antimicrobial peptides (AMPs) are being explored as novel sources of antimicrobial drugs – these small (10-60 residues in length) peptides typically target bacterial membranes and thus have reduced propensity for the development of AMR. Some of the most well-characterised AMPs are derived from bacteria, fungi, insects, and plants, however, this article will shed light on parasitic worms as an unexplored and underappreciated source of AMPs.
The global impact of antimicrobial resistance
Currently, AMR accounts for approximately 4.95 million deaths annually, with projections rising to 10 million per year by 2050 if effective interventions are not implemented. Beyond its direct impact on health, AMR imposes a substantial socio-economic burden, straining healthcare systems and increasing medical costs due to prolonged hospitalisations, the need for more expensive second- and third-line treatments, and increased mortality rates.
The World Bank estimates that AMR could result in a global GDP loss of up to US$100 trillion by 2050, with low- and middle-income countries disproportionately affected due to limited healthcare infrastructure and restricted access to alternative therapies. In these regions, the overuse of antibiotics in both human and agricultural contexts, coupled with inadequate infection control measures, accelerates the emergence and dissemination of resistant strains. Consequently, AMR exacerbates health inequalities, disproportionately affecting vulnerable populations with limited access to effective medical interventions. If left unchecked, AMR could push millions into extreme poverty due to rising healthcare costs and loss of productivity, presenting not only a medical crisis but also a significant global economic and social challenge. This underscores the urgent need for alternative therapeutic strategies to combat multidrug-resistant pathogens.
Drivers of AMR
The primary drivers of AMR include the overuse and misuse of antibiotics, which facilitate the emergence and spread of drug-resistant strains. Resistance mechanisms employed by bacteria include efflux pumps that expel antimicrobial agents, enzymatic degradation, such as β-lactamase production, and biofilm formation that protects bacterial communities from drug penetration. Additionally, the development of new antibiotics has stagnated in recent decades, with pharmaceutical companies facing high research and development costs, long approval processes, and limited financial incentives due to limitations placed on their use (to reduce AMR) and potentially short-term efficacy of new antibiotics before resistance develops. As a result, few novel antibiotics have been introduced to the market, leaving healthcare providers with limited treatment options for MDR infections. This therapeutic gap highlights the urgent need for alternative antimicrobial strategies.

Antimicrobial peptides as a solution
One promising approach to addressing AMR is the development of antimicrobial peptides (AMPs), which offer novel mechanisms of action and a reduced propensity for resistance development. AMPs are naturally occurring molecules and integral components of the innate immune system across a wide range of organisms, including mammals, amphibians, insects, and plants. These small (typically 10-60 residues in length) bioactive peptides exhibit broad-spectrum antimicrobial activity, with some targeting Gram-positive and/or Gram-negative bacteria, while others possess antifungal, antiviral, and antiparasitic activity. Their primary mode of action involves disrupting bacterial membranes through electrostatic interactions, leading to rapid membrane permeabilization and subsequent cell death.
In contrast to conventional antibiotics, which typically target specific bacterial proteins or metabolic pathways, the rapid killing and generic membrane targeting of AMPs make it more difficult for bacteria to develop resistance. Some AMPs may also translocate bacterial membranes, targeting DNA/RNA/protein synthesis or disrupting protein folding. Besides their direct antimicrobial actions, AMPs possess immune-modulatory properties, such as promoting wound healing, reducing inflammation, and modulating cytokine production. Their rapid bactericidal activity, broad target range, and lower likelihood of resistance developing position AMPs as promising alternatives to traditional antibiotics in combating multidrug-resistant infections.
Potential of helminth-derived AMPs
While AMPs from amphibians, insects, and plants have been extensively studied, those secreted by parasitic helminths remain largely unexplored, despite their unique ecological niche and potential biomedical relevance. AMPs from Schistosoma mansonii (causative agent of the neglected tropical disease, schistosomiasis) have been shown to possess membranolytic activity against Gram-positive and Gram-negative bacteria as well as antifungal activity. Some intestinal-dwelling helminths, including members of the Ascaris and Anisakis genera as well as Toxocara canis (a roundworm that infects dogs), have also been demonstrated to produce AMPs. Thanks to these exciting studies, researchers are now beginning to recognise the role of AMPs in modulating host-parasite-microbiome interactions, particularly in species that live in microbe-rich environments, such as intestinal-dwelling helminths. Unlike many of the AMPs discovered in animals and plants, helminth-derived AMPs usually exhibit low or no cytotoxicity towards mammalian cells, likely reflecting the fact that they live in close contact with their host. Helminth-derived AMPs therefore represent a huge untapped source of novel therapeutics.
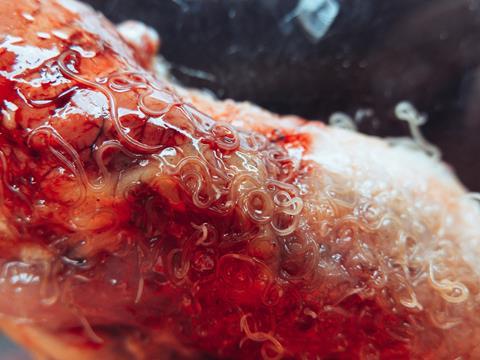
Dr Rebecca Shears (Manchester Metropolitan University) and colleagues at Queen’s University Belfast are currently investigating the antimicrobial and immunomodulatory properties of Trichuris AMPs. Trichuris is a genus of gastrointestinal-dwelling nematodes, with species that infect man (T. trichiura) and animals (the most well-studied being the mouse whipworm, T. muris, and pig whipworm, T. suis). It is well established that Trichuris infections in mice, humans, and pigs lead to altered gut microbiota composition, leading these researchers to hypothesise that worm-derived AMPs could be responsible. Using computational methods, they have identified 5 peptides encoded within the pan-Trichuris genome with potent antimicrobial activity against Gram-negative WHO priority pathogens, including Acinetobacter baumannii and Pseudomonas aeruginosa. One of the peptides also has potent antimicrobial activity against clinical MRSA isolates. Several others were found to exert immunomodulatory activity on macrophage-like cells. Interestingly, these Trichuris-derived AMPs show minimal toxicity against mammalian cells, likely reflecting the evolutionary pressure for these species to cause chronic infections within the host by minimising tissue damage. Perturbing the host’s gut microbiota may provide a more favourable environment for Trichuris species, especially given the complexity of interactions between Trichuris, its host, and the gut microbiota (for example, the requirement for direct contact with colonic bacteria to stimulate egg hatching).
Optimisation of AMPs for therapeutic applications
The optimisation of AMPs for therapeutic applications presents several challenges that must be addressed to ensure their effective use in clinical settings. Key considerations include enhancing their antimicrobial efficacy, minimising cytotoxicity, and improving their in vivo half-life. Although AMPs offer promising therapeutic potential, their application is often hindered by issues such as limited stability, poor bioavailability, and potential toxicity to host cells. Specifically, the selective targeting of pathogenic bacteria whilst minimising toxicity to host cells is critical, as many AMPs can cause damage to mammalian cells at high concentrations, limiting their therapeutic potential. Helminth-derived AMPs may offer an advantage over traditional sources of AMPs in this regard. They may also offer an advantage with regards to stability; since they are released within the host environment, they may have evolved increased resistance to mammalian proteases, although extensive investigations into this have yet to be carried out.
Advances in computational modelling offer new opportunities to address these challenges by facilitating the rational design of AMP structures that optimise antimicrobial properties while simultaneously reducing off-target effects and adverse reactions. Through techniques such as molecular dynamics simulations, docking studies, and predictive algorithms, computational modelling allows the identification of key structural features that enhance peptide stability, specificity, and effectiveness, while mitigating the risks of cytotoxicity and haemolysis.
Outlook and future directions
With only a handful of species having been investigated so far, it seems we have only scratched the surface with regard to helminth-derived AMPs. Production of AMPs may be an important survival strategy for these species, allowing them to modify their environment to promote their own survival. This might include inhibiting microbes that are pathogenic to the worm or perhaps perturbing the microbiota composition to promote a microbial community that aids egg hatching, larval development, or even long-term survival of adult worms. Whatever the biological reason for helminth production of AMPs, they may warrant further investigation as novel antimicrobials, especially given their favourable properties, namely lower toxicity and potentially high stability compared to AMPs from other sources.
Further reading
Global burden of antimicrobial resistance and forecasts to 2050 - The Lancet
Antimicrobial peptides as therapeutic agents: opportunities and challenges
Helminthic host defense peptides: using the parasite to defend the host - ScienceDirect
The scope of the antimicrobial resistance challenge - The Lancet
The economic burden of antibiotic resistance: A systematic review and meta-analysis | PLOS One
Whipworm secretions and their roles in host-parasite interactions | Parasites & Vectors
Innovative Strategies and Methodologies in Antimicrobial Peptide Design







No comments yet