Natural sunscreens shield the skin from harmful radiation, without triggering allergic reactions. In a recently published study, a group of researchers has discovered a novel compound, β-glucose-bound hydroxy mycosporine-sarcosine, which is produced in thermal cyanobacteria under UV-A/UV-B and salt stress.
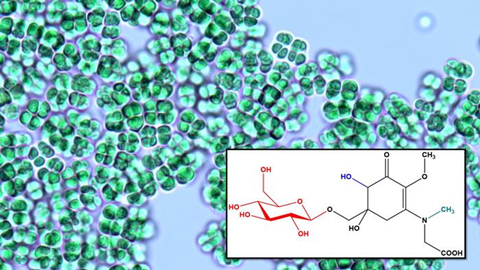
This compound has a unique biosynthesis pathway, which is different from the typical mycosporine-like amino acids (MAAs) biosynthesis mechanism. This discovery aids industrial biotechnology in the production of natural UV-filter compounds.
Cyanobacteria, the oxygen-producing photosynthetic bacteria, survive under extreme conditions. They produce a wide spectrum of primary and secondary metabolites to survive the extreme stressed environmental conditions. Mycosporine-like amino acids (MAAs) are small, water-soluble molecules produced by cyanobacteria that function as ultraviolet (UV)-absorbing compounds. These compounds offer photoprotection and act as antioxidants by scavenging stress-induced reactive oxygen species (ROS). Even though they have a common basic structure, different MAA compounds discovered to date show large structural diversities, which also influence their bioactivity and function.
With a rising risk of harmful UV radiation exposure and skin cancer, researchers are focusing on bioactive compounds with photoprotective abilities. While chemical sunscreens offer the desired protection, they are also associated with allergic reactions and other harmful side effects. MAAs are biocompatible and considered safe for human use, making them immensely important for sustainable biotechnology and large-scale production of natural sunscreen.
Thermal cyanobacteria
In a new study, a team of researchers led by Professor Hakuto Kageyama from Meijo University and Professor Rungaroon Waditee-Sirisattha from Chulalongkorn University has discovered a novel MAA molecule from thermal cyanobacteria inhabiting hot springs in Thailand. The discovery also contributes to understanding the survival strategies of these cyanobacteria, surviving extreme environmental conditions.
“Understanding stress-responsive biosynthesis in extremophilic cyanobacteria may accelerate industrial biotechnology for natural pigment and antioxidant production,” says Prof. Kageyama, while talking about the motivation behind the study. It was made available online on December 01, 2025, and was published in Volume 1009 of Science of The Total Environment on December 20, 2025.
Hot spring
The team isolated eight thermophilic cyanobacterial strains from the Bo Khlueng hot spring in Ratchaburi Province, Thailand. Under experimental setup, the Gloeocapsa species BRSZ strain produced a novel UV-absorbing compound in response to UV-A and UV-B exposures. This compound, identified as β-glucose-bound hydroxy mycosporine-sarcosine (GlcHMS326), was further analyzed for a detailed understanding of its structure and functional mechanism.
This novel compound undergoes triple chemical modifications—glycosylation, hydroxylation, and methylation—which have not been reported in cyanobacteria-derived MAAs before. Genetic analysis showed that these cyanobacteria possess a unique branch of genes that are associated with these modifications.
READ MORE: Natural saclipins in cyanobacteria offer hope of combating skin aging
READ MORE: Scientists reveal how cyanobacteria use ’sunscreen’ to adapt to climate
GlcHMS326 production is strongly induced by UV-A and UV-B irradiation and salt stress. Interestingly, even though the cyanobacteria are obtained from hot water springs, this particular MAA production is not associated with thermal stress. The chemical modifications in GlcHMS326 contribute to its unique structural and functional properties. Methylation can enhance the stability, UV absorption properties, and antioxidant capacity of MAA compounds.
Glycosylation of MAAs has been proposed to enhance their stability and support photoprotection and antioxidative defense. This compound shows higher free-radical scavenging property compared to canonical MAAs, suggesting that the derivatized structure of GlcHMS326 contributes to its enhanced antioxidant potential.
Unique metabolic pathways
The findings of this study contribute to understanding how cyanobacteria living in extreme environments have uniquely evolved metabolic pathways to produce a special natural UV-absorbing substance. This unique MAA plays a pivotal role in abiotic stress tolerance for Gloeocapsa species and likely serves multiple functions in this thermophilic cyanobacteria.
Highlighting the importance of the study, Prof. Waditee-Sirisattha mentions, “Cyanobacteria are deemed unique among the microbial world. Our recent study underscores that extremophilic cyanobacteria are not only ecologically important but also represent a key area of research for multiple disciplines.”
The significance of this compound lies in its diverse functionality and the potential for sustainable, large-scale production using the cyanobacterial “biofactories.” This compound can be utilized as an alternative to certain synthetic UV filters that raise environmental concerns, supporting the development of eco-friendly sunscreens. Its antioxidant activity also hints at its potential applications in anti-aging, skincare, and pharmaceutical formulations.
“This discovery reminds us that nature still holds many chemical surprises. Extremophilic cyanobacteria reveal uncommon molecules that can inspire new directions in basic science and sustainable biotechnology,” concludes Prof. Kageyama.
Topics
- Asia & Oceania
- Bacteria
- Chulalongkorn University
- cyanobacteria
- Economic Equality
- Gloeocapsa species BRSZ
- Hakuto Kageyama
- Meijo University
- mycosporine-like amino acids
- natural sunscreens
- One Health
- Personal Care Product & Cosmetics
- Research News
- Rungaroon Waditee-Sirisattha
- β-glucose-bound hydroxy mycosporine-sarcosine




No comments yet