Promising ethers
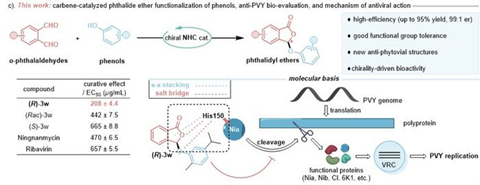
Prof. Runjiang Song’s team at the State Key Laboratory of Green Pesticide, Guizhou University, reports a carbene-catalyzed phthalide ether functionalization of phenol compounds, facilitating facile synthesis of a variety of optically-enriched aryl phthalidyl ethers (Fig. 1).
A myriad of differently substituted phenols, even natural phenols, can be converted into their corresponding products with outstanding enantioselectivity (up to 99:1 er) and conversion rates (up to 95% yield) under an optimal condition catalyzed by N-heterocyclic carbene (NHC). The team further discovered that the phthalide ether derivative (R)-3w of carvacrol possesses potent activity against PVY through bio-evaluations. Specifically, it displayed substantially superior and enantioselectivity-preferred curative effects in comparison to the popular commercial drugs ningnanmycin and ribavirin, as well as its enantiomer (S)-3w and racemic mixture (Rac)-3w, which encouraged subsequent mechanism of action investigations.
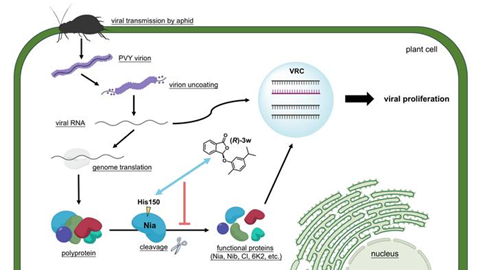
It is noteworthy that the curative effect of a drug usually correlates with its impact on pathogen replications, and the current mechanisms of antiviral action in plants are primarily based on the protective and inactivating activities of virucides. Combining molecular docking with a series of molecular biological validations allows reasonably hypothesize that (R)-3w may act on a brand-new molecular target via His150 residue, namely the Nia involved in cleavage of the viral polyprotein into functional proteins.
Several of these proteins are essential for generation of the viral replication complex (VRC), which mediates PVY replication. Both the mutation at site 150 and (R)-3w treatment significantly reduced the proliferation of PVY in Nicotiana benthamiana (N. benthamiana). This study contributes novel antiviral structures, asymmetric synthetic protocol, and potential new target protein for the PVY management.
Future prospects
The core innovation of this research lies in the utilization of NHC catalysis to achieve the phthalide functionalization of a variety of natural phenolic compounds. This method not only boasts good yields and enantioselectivity but also exhibits excellent functional group tolerance.
Experimental results indicate that (R)-3w demonstrates significant antiviral activity against PVY, with its antiviral efficacy being markedly different from that of the racemic mixture (Rac)-3w and the enantiomer (S)-3w, and superior to that of the existing commercial antiviral agents Ningnanmycin and Ribavirin. Fig. 2 illustrates the antiviral mechanism of (R)-3w: (R)-3w binds to the His150 residue of the Nia protein of PVY, potentially disrupting the normal cleavage of the viral polyprotein, reducing the functional proteins available for the formation of the viral replication complex (VRC), and thereby inhibiting viral proliferation.
This study not only provides a theoretical basis for the prevention and control of plant viruses but also offers new synthetic methods and potential targets for the development of novel phytovirucides.






No comments yet