Efficient intercellular viral spread is critical for pathogenesis and virulence. In a study published in Science Bulletin, researchers from Peking University Health Science Center and Harbin Veterinary Research Institute found that in infected cells, nucleic acids and proteins of vesicular stomatitis virus (VSV) are actively transported into migrasomes, newly characterized cellular structures generated specifically during cell migration.
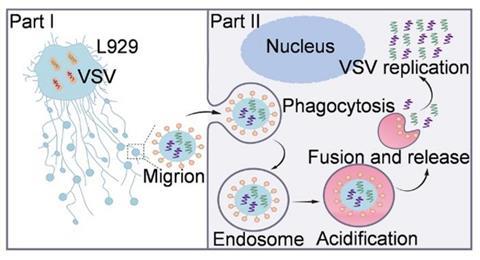
Migrasomes with viral nucleic acids inside and VSV-G on the surface were named “Migrions”, which are large virus-like structures distinct from free VSV particles. Migrion-mediated VSV transmission exhibits accelerated replication kinetics compared to free virions, attributed to the collective delivery and simultaneous replication of multi-copy viral genomes within single host cells.
Notably, Migrions facilitate co-transmission of heterologous viruses, a mechanism distinct from conventional extracellular vesicle (EV)-mediated viral dissemination.
Mechanistically, Migrions enter recipient cells via endocytosis in a receptor-independent manner. Under acidic conditions, VSV-G on Migrions triggers membrane fusion with endosomes, releasing viral cargo, which is essential to initiate replication. In murine infection models, Migrions demonstrated significantly enhanced infectivity and pathogenicity relative to free virions, causing severe pulmonary and cerebral infections characterized by encephalitis and lethal outcomes.
The researcher proposed that “Migrion”—a chimeric structure formed between virus and migrasome—represents a novel paradigm of intercellular viral transmission, one that directly couples viral dissemination with cell migration. This discovery challenges conventional models of viral spread by introducing a migration-dependent mechanism that exploits the host’s migratory machinery for systemic infection.






No comments yet