Chronic inflammatory diseases are increasingly recognized as being tied to systemic metabolic dysfunction, overturning the old belief that local infections stay confined. Research now shows that inflammation arising in a single site like the gums or the root of a tooth can send systemic signals that disrupt glucose regulation.
Oral diseases, once dismissed as dental issues, have become central to this evolving picture. Among the key culprits is Porphyromonas gingivalis (Pg), a pathogen behind gum disease and periapical lesions. With its atypical lipopolysaccharide (LPS) and strong inflammatory drive, Pg has emerged as a notable link between persistent oral inflammation and broader metabolic imbalance.
READ MORE: Heart rhythm disorder traced to bacterium lurking in our gums
READ MORE: Gargling away the bad bacteria in type 2 diabetes can help to control blood sugar
To investigate this possibility, Pr. Vincent Blasco-Baque and his research team at INSERM/Université de Toulouse conducted a comprehensive study, recently published in Volume 17 of the International Journal of Oral Science on 13 November 2025. Their goal was to determine whether Pg-driven periapical lesions could influence systemic metabolism through IL-17–mediated inflammation. Building on earlier findings linking gut bacteria and LPS to metabolic disease, the team sought to uncover whether similar mechanisms could originate from the oral cavity, specifically from endodontic infections.
Periapical tissues
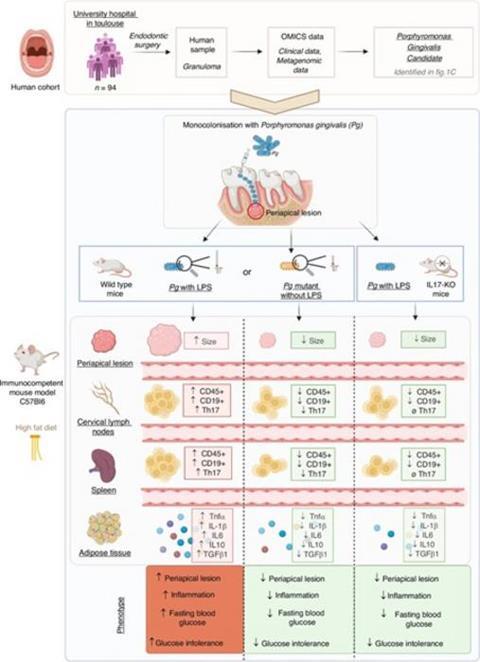
The researchers first analyzed periapical tissues from 94 endodontic surgery patients and found Pg to be a predominant pathogen, particularly in severe lesions.
Vincent Blasco-Baque explains: “To test causation and mechanism, we developed a controlled mouse model in which the endodontic space was monocolonized with Pg. Mice were placed on a high-fat diet to simulate metabolic stress and were assigned to receive either wild-type Pg with intact LPS, a weakened LPS strain, or purified LPS alone.”
An additional group lacked IL-17 to determine its role in disease progression. Across all groups, the team evaluated periapical bone loss with micro-CT, profiled immune activation, and assessed adipose inflammation, dysbiosis, and glucose metabolism.
Link to metabolic dysfunction
These findings reveal a clear mechanistic link between Pg-induced periapical disease and systemic metabolic dysfunction. As Vincent Blasco-Baque shares, “Mice exposed to Pg with functional LPS showed marked periapical bone loss and a surge in Th17 cells and IL-17.”
He further notes, “These effects extended systemically: in high-fat–diet mice, Pg-LPS significantly worsened glucose intolerance, accompanied by adipose dysbiosis, inflammation, and cytokines that disrupt insulin signaling.”
In IL-17 knockout mice, however, this cascade largely disappeared. Pg or its LPS failed to induce substantial bone loss, metabolic impairment, or inflammatory activation. Without IL-17, Pg lost much of its capacity to drive local and systemic pathology.
Periapical lesions
This study offers several key benefits and insights. It highlights a previously underrecognized role of periapical lesions in influencing systemic metabolic health. It also establishes a robust causal model rather than solely correlational evidence. Finally, it identifies IL-17 as a potential therapeutic target, suggesting that modulating Th17 activation or blocking Pg virulence factors, such as gingipains or O-antigen LPS components, may reduce both local tissue destruction and systemic metabolic risk.
“Looking ahead, these findings open opportunities for novel interventions, including IL-17 inhibitors, gingipain blockers, microbiome-modulating approaches, and improved diagnostic biomarkers to assess systemic impact of endodontic infections.” shares Vincent Blasco-Baque. Further research into synergistic interactions with other oral pathogens may also deepen understanding of oral-systemic disease links.
In conclusion, this study demonstrates that Pg and its LPS are potent drivers of both periapical bone destruction and systemic metabolic dysfunction, acting through an IL-17–dependent inflammatory pathway. By unveiling an oral–systemic axis connecting endodontic infection to metabolic disease, the work lays the foundation for new therapeutic strategies aimed at protecting both oral and systemic health.







No comments yet