Sulphur is a fundamental element of life and all organisms need it to synthesize cellular materials. Autotrophs, such as plants and algae, acquire sulphur by converting sulphate into sulphide, which can be incorporated into biomass.

However, this process requires a lot of energy and produces harmful intermediates and byproducts that need to be immediately transformed. As a result, it was previously believed that microbes known as methanogens, which are usually short on energy, would be unable to convert sulphate into sulphide. Therefore, it was assumed that these microbes, which produce half of the world’s methane, rely on other forms of sulphur, such as sulphide.
This dogma was broken in 1986 with the discovery of the methanogen Methanothermococcus thermolithotrophicus, growing on sulphate as the only sulphur source. How is this possible, considering the energetic costs and toxic intermediates? Why is it the only methanogen that seems to be capable of growing on this sulphur species? Does this organism use chemical tricks or a yet unknown strategy to allow sulphate assimilation?
Marion Jespersen and Tristan Wagner at the Max Planck Institute for Marine Microbiology have now found answers to these questions in a study published in the journal Nature Microbiology.
Switching to sulphate
The first challenge the researchers met was to get the microbe to grow on the new sulphur source. “When I started my PhD, I really had to convince M. thermolithotrophicus to eat sulphate instead of sulphide,” says Marion Jespersen. “But after optimizing the medium, Methanothermococcus became a pro at growing on sulphate, with cell densities comparable to those when growing on sulphide.”
“Things got really exciting when we measured the disappearance of sulphate as the organism grew. This was when we could really prove that the methanogen converts this substrate.”
This allowed the researchers to safely cultivate M. thermolithotrophicus in bioreactors in large scales, as they were no longer dependent on the toxic and explosive hydrogen sulphide gas for growth. “It provided us with enough biomass to study this fascinating organism,” explains Jespersen. Now the researchers were ready to dig into the details of the underlying processes.
Sulphate assimilation pathway
To understand the molecular mechanisms of sulphate assimilation, the scientists analyzed the genome of M. thermolithotrophicus. They found five genes that had the potential to encode sulphate-reduction-associated enzymes.
“We managed to characterize every one of those enzymes and therefore explored the complete pathway. A true tour de force when you think about its complexity,” says Tristan Wagner, head of the Max Planck Research Group Microbial Metabolism.
By characterizing the enzymes one-by-one, the scientists assembled the first sulphate assimilation pathway from a methanogen. While the first two enzymes of the pathway are well known and occur in many microbes and plants, the next enzymes were of a new kind.
Surprising discovery
“We were stunned to see that it appears as if M. thermolithotrophicus has hijacked one enzyme from a dissimilatory sulphate-reducing organism and slightly modified it to serve its own needs,” says Jespersen. While some microbes assimilate sulphate as a cellular building block, others use it to obtain energy in a dissimilatory process – as humans do when respiring oxygen.
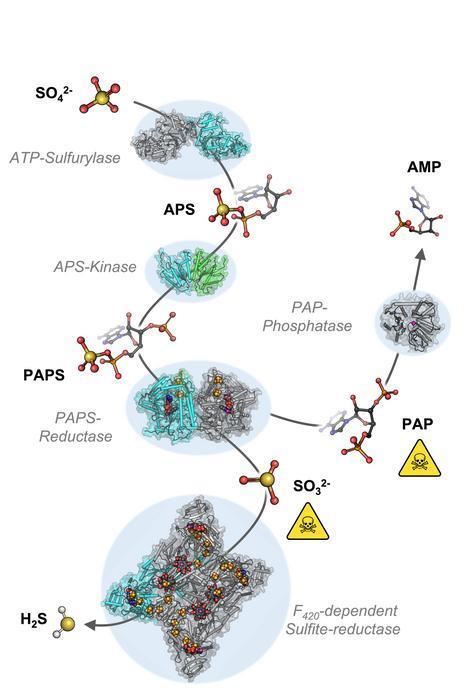
The microbes that perform dissimilatory sulphate-reduction employ a different set of enzymes to do so. The methanogen studied here converted one of these dissimilatory enzymes into an assimilatory one.
“A simple, yet highly effective strategy and most likely the reason why this methanogen is able to grow on sulphate. So far, this particular enzyme has only been found in M. thermolithotrophicus and no other methanogens,” Jespersen explains.
Dealing with poisons
However, M. thermolithotrophicus also needs to cope with two poisons that are generated during the assimilation of sulphate. That´s what the last two enzymes of the pathway are made for: The first one, again similar to a dissimilatory enzyme, generates sulphide from sulphite. The second one is a new type of phosphatase with robust efficiency to hydrolyze the other poison, known as PAP.
“It seems that M. thermolithotrophicus collected genetic information from its microbial environment that enabled it to grow on sulphate. By mixing and matching assimilatory and dissimilatory enzymes, it created its own functional sulphate reduction machinery,” says Wagner.
New avenues
Hydrogenotrophic methanogens, such as M. thermolithotrophicus, have the ability to convert dihydrogen (H2, for example artificially produced from renewable energy) and carbon dioxide (CO2) into methane (CH4). In other words, they can convert the greenhouse gas CO2 into the biofuel CH4, which can be used, for example, to heat our homes.
To do this, methanogens are grown in large bioreactors. A current bottleneck in the cultivation of methanogens is their need for the highly hazardous and explosive hydrogen sulphide gas as a sulphur source. With the discovery of the sulphate-assimilation pathway in M. thermolithotrophicus, it is possible to genetically engineer methanogens that are already used in biotechnology to use this pathway instead – leading to safer and more cost-effective biogas production.
“An unresolved burning question is why M. thermolithotrophicus would assimilate sulphate in nature. For this, we will have to go out into the field and see if the enzymes required for this pathway are also expressed in the natural environment of the microbe,” concludes Wagner.




No comments yet