An international research team has identified potential new ‘weapons’ in the ‘arms race’ for new antibiotics and possible future therapies for a more balanced gut microbiome and human health.

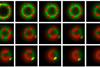
Using DNA sequencing, research led by Flinders University has singled out a few tiny gut viruses, from among hundreds of common Crassvirales ‘bacteriophages’ or phages found in the human digestive system that target specific bacterial hosts.
This paves the way for ‘phage therapy’ to target ‘bad’ or unbalanced bacteria in the gut – so important for human digestion and nutrition, immunity and general health and wellbeing – along with possible solutions to antimicrobial resistance (AMR) when broad-spectrum antibiotics no longer work on these bacteria.
Global threat
The WHO has warned that AMR is one of the top public health threats facing humanity in the 21st century and was associated with the death of close to 5 million people in 2019. If no action is taken it could cost world economies upwards of US$1 trillion by 2050. Multiple genetic changes in common bacteria can lead them to become resistant to multiple antibiotics, forming what’s called ‘superbugs’.
“Our study is a significant step forward in the emerging field of phage therapy,” says Flinders University researcher Bhavya Papudeshi, from the Flinders Accelerator for Microbiome Exploration (FAME) Lab, about a new article in Microbial Genomics.
“By understanding how these few Crassvirales species specifically prey on Bacteroides species within the human gut, we unveil a novel and intricate interaction between phage-host and infection mechanisms,” she says.
“This complex detail of adaptation and diversity in the gut paves the way for more effective and sustainable phage therapy, where we can use specific bacteriophages to infect and eliminate targeted bacteria.”
Phage therapy
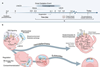
Phage therapy can be a more precise way to fight dangerous infections when the right phage is found to kill harmful or targeted bacteria while leaving beneficial bacteria unharmed. Most antibiotics kill both beneficial and harmful bacteria which can lead to imbalance in the gut microbes.
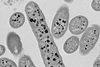
Bacteroides are mainly beneficial bacteria in the gut because they metabolise polysaccharides and oligosaccharides which provide important vitamins and nutrition to the body and other intestinal microbial residents.
However when gut balance is lost, they can cause problems with pathogenic strains, compromised immunity, excessive dietary fibre consumption and antibiotic resistance.
Untangling interactions
Research coauthor, FAME Lab leader Flinders University Professor Robert Edwards, says: “Our research efforts are starting to untangle some of the complex interactions between the hundreds of thousands of bacteria species and phages, the microbes which can either co-exist with bacterial hosts or infect and ‘clean up’ certain bacteria.
“In this case, Bacteroides bacteria are also key in breaking down complex polysaccharides that otherwise would remain undigested – so their numbers also hold a key to metabolism and other aspects of gut health.
“These latest discoveries may lead to new methods of improving gut health and metabolism to support overall wellbeing.”
The study, supported by researchers in Australia, the US and Europe, used samples from wastewater to isolate three novel Crassvirales species that infect Bacteroides cellulosilyticus WH2, belonging to different genera and families. This confirmed these phages have a shared ability to exploit similar features in their bacterial host – using a tail spike protein to infect the same bacterial host.
Find out more about the project at ‘Bacteria assassins’ in the new Fearless Research series here.




No comments yet