The World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) estimated that, as of 2020, 2 billion people across the globe did not have access to safely managed drinking water.
Ideally, safely managed drinking water should be available onsite, from an improved water source and ‘not represent any significant risk to health over a lifetime of consumption, including different sensitivities that may occur between life stages’ (WHO and UNICEF, 2021). Strictly, this requires no detectable potential pathogens such as Escherichia coli or coliforms in drinking water. Globally we estimate that 43% of people live in rural areas and only 60% of these rural populations have access to safely managed drinking water services. For rural communities, or communities in low-middle income countries where the GDP per capita is less than US$ 2581, unrestricted access to safely managed drinking water is challenging due to costs associated with centralised drinking water treatment systems and necessary distribution networks. If the UN Sustainable Development Goal 6 ‘Clean Water and Sanitation for All’ is to be fully realised by 2030, more innovative and transformative decentralised solutions that are applicable to resource-limited settings are urgently required.
Stored freshwater can be a lifeline for millions of people who live in water-stressed or water-scarce environments. The frequency and associated hazards of extreme weather events, (droughts and flooding) are increasing. In response, many communities rely on water from stored supplies. This water can be stored for many months, resulting in water quality deterioration over time, increasing the potential for the presence and accumulation of harmful waterborne pathogens and the proliferation of biofilms on storage infrastructure. Consequently, the WHO recommends treating stored water prior to consumption, wherever possible, by filtration, boiling or chlorination to ensure the control of waterborne pathogens.
Prior to any drinking water disinfection process, it is suggested that source water is filtered to remove sediment and organic material that can interfere with disinfection processes. It has long been demonstrated that the presence of organic matter can negatively impact disinfection efficacy and result in the formation of hazardous disinfection by-products, such as haloacetic acids and trihalomethanes. Filtering source water can be undertaken using simple methods such as sand and ceramic filters or using commercial microfiltration or ultrafiltration (UF) membranes. Sand and ceramic filters are both low-cost (sand and/or clay is readily available and accessible in most parts of the world) and simple constructs, such that they can be universally produced using limited infrastructure and resources. Commercially manufactured fine-filtration membrane systems, such as UF, have the potential to deliver high-quality drinking water at scale but are more expensive to procure, install and maintain. All filter-based systems are prone to biofouling caused by the build-up of organic material and biofilms. Biofilm fouling drastically reduces performance and will either require mechanical (for sand filtration) of chemical (for UF) cleaning. As a result, sand filtration systems are often implemented in resource-limited settings as commercial filtration membrane systems are often viewed as infeasible or impractical options.
We are undertaking research that focuses on the ‘decentralised management and control’ of community-scale water supplies using electrochemical technologies, as well as new scalable, nature-based treatment systems for the low-cost provision of safe drinking water.
Point-of-use electrochemical water disinfection
The disinfection of potable water can be achieved using chemical or physical processes. For chemical disinfection, substances commonly used to treat water include ozone, chlorine, sodium hypochlorite or chlorine dioxide. These chemical approaches have been shown to be reliable and effective, whilst some also provide a disinfection residual that maintains a disinfection reservoir, which is necessary if safety is to be maintained throughout distribution networks. A key disadvantage of chemical disinfection is the production of unwanted and dangerous disinfectant by-products, which can accumulate in the distributed water supply. In addition to this, there are also hazards in producing, transporting and handling large amounts of chemical substances such as chlorine and ozone. Globally, the use of chlorine (Cl2) and related substances, including hypochlorite (OCl−) and chlorine dioxide (ClO2), are by far the most common disinfectants used for treating drinking water supplies, primarily due to their relatively low cost and wide availability. Attempts to produce chlorine and chlorine-related substances by direct electrolysis of brine had been reported as early as the nineteenth century, and since the end of the nineteenth century there have been numerous attempts to develop and use electrochemical disinfection, with limited success until recently, whereby electrochemical water disinfection technologies have reached technical and scientific maturity. Technical advancements have been made through the successful fabrication of sufficiently stable and efficient titanium electrodes with mixed oxide coatings based on iridium and/or ruthenium oxide materials, in combination with recent scientific breakthroughs relating to: (1) the functional interrelationships between the chloride concentration in the water, the current, the current density and the new electrode materials; and (2) the electrochemical production of a variety of chlorine species and their respective disinfecting actions and efficacies.
The ability to produce efficacious disinfectants in situ, via direct electrolysis, minimises the need to transport and store hazardous chemicals, thus reducing the potential for accidental chemical release into the environment. Electrochemical generators that have a semi-permeable membrane separating the anodic and cathodic cells produce two solutions: chlorine-based antimicrobial (anolyte) and a surfactant or degreasing solution (catholyte). The disinfectants HOCl and OCl− ions are produced at the anode as a side reaction to oxygen evolution. In simplistic terms this occurs as chlorine is first produced electrochemically from dissolved chloride ions. This produced chlorine hydrolyses in the water to form HOCl, which in aqueous solution partially dissociates into the anion OCl− in a pH-dependent equilibrium. The uses and limitations of OCl− are well understood; however, knowledge associated with the antimicrobial properties and uses of HOCl is much more limited. The sum of HOCl and OCl− concentrations is termed ‘free chlorine’ or ‘active chlorine’ and this value defines the disinfection power of any given solution. Despite being relatively easy to make, it is difficult to maintain a stable HOCl solution. In the last 20 years, scientists have been able to cost-effectively produce and maintain stable HOCl for commercial uses. More so, advanced HOCl solutions, produced via state-of-the-art electrochemical systems, have emerged as a safe and viable disinfectant for point-of-use (POU) applications. This is important as HOCl is now known to be superior to OCl− in terms of its antimicrobial efficacy, biocompatibility (important for UF membranes), human toxicity and its lower potential to form hazardous by-products when compared to OCl− (Clayton et al., 2019 ). Our recent research has also shown that electrochemically generated HOCl exhibits significant higher antibiofilm activity than OCl− solutions and that this technology can be successfully integrated into POU drinking water treatment infrastructure. Pre-dosing of UF membranes with electrochemically generated HOCl helps to manage and mitigate biofilm formation, whilst post-dosing of ultrafiltrate water maintains disinfection of produced drinking water. Pilot trials at the University of the West of England (UWE), Bristol have successfully demonstrated a novel off-grid drinking water production system integrating electrochemically generated HOCl and UF membranes. The trial has resulted in approximately 36 months of continuous operation and the production of over 5 million litres of safe drinking water.

In situ or direct electrochemical activation (ECA) technologies do not have a membrane separating the anode and cathode and they have the benefit of utilising low levels of salts and ions that are naturally present in freshwater sources such as rainwater and groundwater. Electrochemical reactions occurring at the anode have been shown to transform dissolved chloride ions present in rainwater into measurable free or active chlorine to the extent that these activated rainwater solutions exhibit antimicrobial properties. Such technologies have the advantage of being scalable, inexpensive (typically between $50 and $200), simple in design and with low maintenance and power requirements. Such innovations are suitable for deployment in low-resource settings and could be used to treat stored freshwater supplies. Recent research at UWE has demonstrated that biologically safe water (coliform-free) can be achieved using a prototype direct ECA unit, via the electrolysis of naturally occurring ions that are present within rainwater. Initial operational periods of up to 7 days have been piloted in the UK and these demonstrate that when in continuous operation, potential pathogens (total coliforms) can be reduced to zero CFU/100 mL. Recent field-based case studies of these small-scale in situ ECA technologies, in collaboration with charitable partners Frank Water and Bala Vikasa, occurring in Telangana, India and Sadabe in Tsinjoarivo, Madagascar have demonstrated that this approach negates, or significantly reduces the need for post-collection treatment. These international trials have demonstrated that in situ ECA technologies can significantly reduce total viable bacteria and completely remove total coliforms and E. coli, reducing the risk of preventable diseases.

Harnessing the power of biofilms
Biofilms are communities of microorganisms that exist in almost every environmental niche, including our own bodies. We have been harnessing these biofilms through first maturing them on inert ceramic substrates, whereby multispecies environmental microorganisms from aquatic systems are allowed to attach, colonise and form mature biofilms. Our recent research demonstrates that these mature biofilms can reduce and help control planktonic bacterial pathogens such as E. coli and enterococci when used to treat contaminated water sources, and furthermore that this phenomenon is as a result of biological action alone (Steven et al., 2022). This is distinct from current standard treatment approaches that apply physical exclusion (filtration) or disinfection methods to reduce the number of planktonic pathogens. Engineering biofilm-based water treatment systems in this way can be related to biofilms observed in nature. The biofilms present in the guts of humans and animals act as a natural inherent defence against ‘foreign’ or pathogenic bacteria, and we show that this natural process can be engineered within fresh water treatment systems.
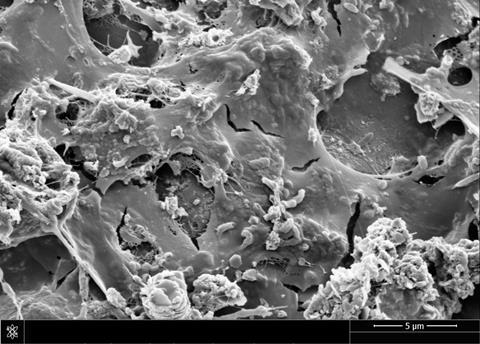
As part of our study, we investigated the significance of the ceramic properties used to accommodate the biofilm. To date, our research found that the porosity or changes in surface area of the ceramics did not have a significant effect on observed efficiencies of the biofilm treatment systems. Clay is an abundant and inexpensive resource and is easily modified through a variety of physical, chemical and thermal treatments. The manipulation of clay and ceramics by communities is widespread across the globe, resulting in a mosaic of clay and ceramic products. This research raises the prospect of utilising low-cost, locally manufactured systems for the control of pathogens in drinking water supplies using an abundant material (ceramic) that is locally available. This negates the need for specialist materials or operational expertise and also removes issues relating to complex supply chains.
Diseases that are associated with poor drinking water quality, sanitation and hygiene services (e.g. diarrhoea) are easily preventable through a variety of low-cost interventions. Yet, in order to achieve the 2030 Sustainable Development Goal 6.1 targets of universal and equitable access to safe drinking water for all, the pace of progress needs to increase by at least four times. If the pace of progress does not increase, it is estimated that by 2030, 1.6 billion people will be without access to safely managed drinking water services, 2.8 billion won’t have access to safely managed sanitation services and 1.9 billion won’t be able to access basic hygiene services. Some of the work reported here highlights reasons for optimism in our ability to achieve Sustainable Development Goal 6.1 by 2030.
Funding sources of research projects including within this article include Natural Environment Research Council, UK [NE/R003106/1] and UK-Africa GCRF Agri-tech Catalyst Seeding Award (Reference: 6675946), as well as Origin Aqua Technologies, Centrego Ltd and Portsmouth Aqua Ltd.








No comments yet