In the microbiology laboratory, we observe infection in real-time: bacterial colonies spreading across agar plates, inflammatory markers rising in blood samples, and immune responses captured at single timepoints. But what if we could watch only one frame at a time of an entire infection unfold from initial pathogen invasion through host immune response to terminal disease progression, all preserved across several hosts? This is precisely what fossilized bone offers microbiologists: a geological-scale petri dish where host-pathogen dynamics are frozen in mineralized tissue, waiting to be decoded.
Our recent study in The Anatomical Record documents six dinosaurs from a single Upper Cretaceous locality in Southeast Brazil (the Ibirá site), all exhibiting osteomyelitis, an inflammatory bone condition typically caused by bacterial, protozoal, fungal, or viral pathogens. This concentration of infections at one site over a geologically constrained timeframe suggests something paleontologists rarely encounter: evidence of a possible ancient epidemic event captured in the fossil record.
The Ibirá fossil assemblage
Between 2006 and 2023, excavations at the “Vaca Morta” site in São Paulo’s backcountry unearthed six medium-to-large bone fragments, including zygopodia (lower limb bones) and ribs, from the São José do Rio Preto Formation, dated to approximately 80 million years ago. The specimens consist of titanosaurs, the latest-surviving group of long-necked dinosaurs. What makes this assemblage remarkable is not merely the presence of pathology, but its prevalence: osteomyelitis cases in sauropods remain exceedingly rare in the global fossil record, making this concentration statistically anomalous.

For microbiologists, these specimens represent something unprecedented in infectious disease research, a nearly complete preservation of inflammatory cascades without the temporal limitations of clinical observation. Unlike modern pathological samples, where we capture disease at discrete moments, fossilized bone records the entire progression: from initial periosteal breach through vascular proliferation, immune-mediated bone remodeling, and ultimately, systemic failure leading to death.
Diagnosing infection across deep time
Modern diagnostic microbiology relies on culturing pathogens, detecting antigens, or identifying genetic material. Such approaches are impossible with 80-million-year-old specimens where soft tissues and microorganisms have long since degraded. Instead, we must read the host’s response, interpreting bone remodeling patterns as proxy evidence for microbial activity.
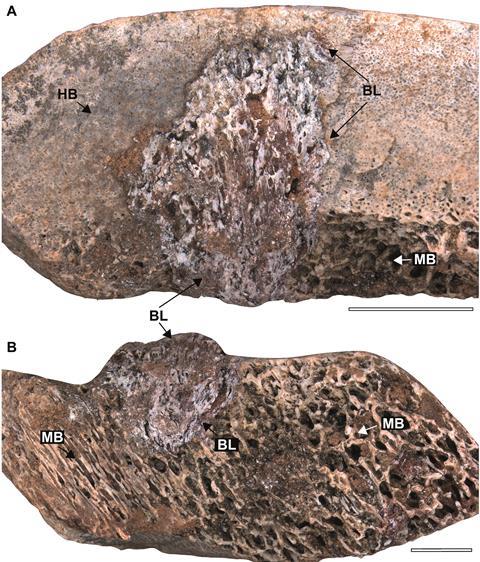
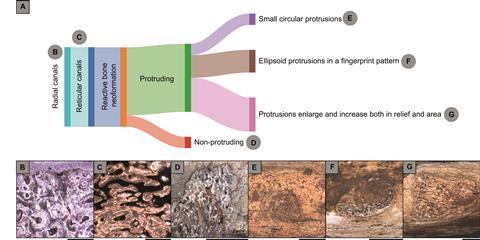
Our analysis revealed three distinct morphological signatures of periosteal reaction and reactive bone neoformation. The first pattern manifested as small circular protrusions on bone surfaces, likely representing localized infection sites where pathogens breached the periosteal membrane (specimen LPP-PV-213). The second pattern displayed ellipsoid protrusions arranged in what Bruce Rothschild termed a “fingerprint pattern,” suggesting lateral infection spread along vascular pathways (specimens LPP-PV-211 and 212). The third category comprised enlarged protrusions extending both vertically and laterally, indicating advanced disease with extensive tissue involvement (specimens LPP-PV-210 and 214).
One specimen (LPP-PV-209) exhibited pathology restricted to the medullary cavity with centrifugal progression through cortical bone toward, but not reaching, the periosteum. This mirrors the pathophysiology of acute hematogenous osteomyelitis in modern vertebrates, where infection typically originates in the metaphysis or medullary space, then spreads through Haversian and Volkmann canals to reach the periosteal surface.
The affected areas exhibited characteristic spongy, “microbubbly” textures, telltale signs of neovascularization accompanying inflammatory response. This vascular proliferation, readily identifiable even in fossilized material, represents the host’s attempt to increase blood flow for delivering immune cells and nutrients to the infection site, a fundamental response conserved across vertebrate evolution.
Histological evidence: reading bone architecture as microbial record
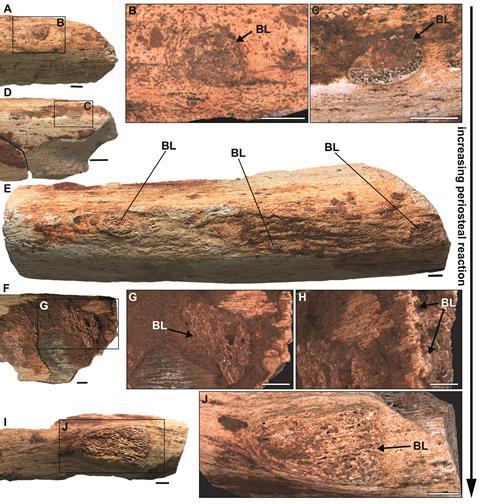
Histological examination revealed avascular, highly fibrous bone matrix organized into thick bundles with chaotic, multidirectional orientation within delimited cortical zones. This tissue architecture is pathognomonic for osteomyelitis, distinguishing it from neoplastic conditions like osteosarcoma that produce superficially similar gross morphology. The reactive bone exhibited decreased bone volume density compared to adjacent cortical tissue (area covered by bone versus area occupied by vascular canals and resorption cavities), contrasting sharply with the increased density observed in some occurrences of osteosarcoma and tuberculosis-like lesions.
This highly vascularized tissue results from osteoblastic bone neoformation in an intensive osteogenic scenario. The dome-shaped lesions in cross-section connected the medulla to the outer cortex, a distribution pattern inconsistent with osteoarthropathy (which localizes to articulation zones) or neoplastic growths.
Most critically, none of the six specimens showed evidence of healing tissues. We found no sequestra (fragments of dead bone), no involucra (new bone sheaths encasing infection), and no cloacae (drainage pathways for purulent material), structures routinely observed in chronic osteomyelitis cases that progress toward resolution. This complete absence of reparative responses indicates these infections remained active at death; these animals succumbed while battling systemic infection, likely experiencing what we would today recognize as septic progression.
Paleoecological context: environmental microbiology of the Cretaceous
Understanding why Ibirá became an infection hotspot requires reconstructing its ancient microbial ecology. During the Late Cretaceous Santonian stage, this region experienced arid climate conditions punctuated by shallow, sluggish rivers and extensive pools of standing water. From a microbiological perspective, these environmental parameters would have created ideal circumstances for pathogen proliferation and transmission.
Stagnant water bodies in warm climates function as microbial reservoirs where bacterial populations reach high densities, particularly for facultative anaerobes and waterborne pathogens. The diverse fauna documented at Ibirá, including turtles, crocodile-like organisms, several types of carnivorous dinosaurs alongside titanosaurs, suggests these water sources attracted multi-species congregation, facilitating zoonotic pathogen circulation through both direct contact and indirect environmental transmission.
We propose two non-mutually exclusive transmission scenarios grounded in modern infectious disease ecology. First, vector-borne transmission via hematophagous arthropods: our previous work documented blood parasites in Ibirania parva, a smaller titanosaur from this same locality, suggesting that blood-feeding vectors could have been active in this ecosystem. Mosquitoes or similar vectors could have introduced pathogens directly into the circulatory system, potentially leading to hematogenous osteomyelitis when microbes colonized bone tissue.
Second, waterborne transmission through ingestion of contaminated water could have caused gastrointestinal infection with subsequent hematogenous spread to skeletal elements. This route parallels modern Salmonella infections in reptiles, where enteric pathogens breach intestinal barriers and disseminate systemically via the bloodstream.
The temporal and spatial clustering of these six cases strongly argues for an epidemic event rather than sporadic infections. This pattern suggests environmental conditions persistently favored pathogen survival and transmission, possibly across multiple seasons or years, rather than isolated introduction events.
Comparative pathology: evolutionary conservation of immune response
One of the most striking findings from our analysis is how closely the dinosaurian immune response mirrors modern vertebrate pathophysiology. The periosteal reaction, characterized by reactive bone formation in response to infection, follows identical basic mechanisms we observe in contemporary clinical osteomyelitis. This evolutionary conservation indicates that fundamental aspects of vertebrate immunology, particularly inflammatory cascades and tissue repair mechanisms, have remained functionally unchanged over 80 million years.
The progression patterns documented in these specimens parallel modern clinical presentations with remarkable fidelity. Acute osteomyelitis in extant species begins in the metaphysis or medullary cavity, spreads through vascular canals, causes cortical breakdown and periosteal elevation, precisely the sequence preserved in our fossil specimens.
Modern vertebrates occasionally resolve osteomyelitis through sequestrum formation (walling off dead bone) and immune-mediated bacterial clearance, processes that leave recognizable histological traces. The absence of such healing markers in all six Ibirá specimens indicates either overwhelmingly severe infections, compromised host immune function, or rapid disease progression precluding effective immune response.
Inferring the etiological agent
While we cannot definitively identify the specific pathogen responsible, bacteria, viruses, fungi, and protozoa all can cause osteomyelitis and are rarely preserved in fossil material, the host response patterns offer clues about the likely culprits. The severity of bone destruction, the extensive periosteal reaction, and the rapid progression without healing most closely resemble pyogenic bacterial infections in modern comparative cases. Based on modern analogues, the most likely etiological agents would be bacterial species analogous to contemporary Staphylococcus aureus (which causes aggressive osteomyelitis via hematogenous spread) or enteric pathogens like Salmonella species (commonly associated with reptilian osteomyelitis following gastrointestinal infection). The absence of granulomatous reaction patterns makes mycobacterial infections (tuberculosis-like disease) less probable.
The relatively rapid disease progression suggested by the absence of sequestra, involucra, and cloacae indicates either highly virulent pathogens or immunocompromised hosts, or both. This rapid advancement contrasts with chronic osteomyelitis cases described in other dinosaur specimens, where extensive healing and bone remodeling occurred before death.
Implications for microbial evolution and disease ecology
This research extends microbiological understanding beyond the temporal constraints of modern experimental systems. These fossils demonstrate that host-pathogen interactions observable in deep time reveal fundamental principles of infection biology transcending species and geological epochs.
The apparent epidemic nature raises intriguing evolutionary questions relevant to modern microbiology. Did these infections represent novel pathogen strains adapted to exploit specific environmental conditions at Ibirá? Or did ecological stressors (congregation around limited water sources, nutritional deficiency, or environmental contamination) simply increase population susceptibility? These questions parallel contemporary concerns about emerging infectious diseases and environmental influences on disease dynamics.
The findings also underscore an often-overlooked reality: even the largest terrestrial vertebrates ever to exist remained profoundly vulnerable to microscopic pathogens. Body size offered no protection against microbial invasion; if anything, the extensive vascular networks required to support multi-ton body masses may have facilitated hematogenous pathogen dissemination.

A window into deep-time microbiology
The Ibirá osteomyelitis cases offer microbiologists tangible evidence that infectious diseases have shaped vertebrate evolution for at least 80 million years. These bones serve as archives of microbial activity from an era when flowering plants were just diversifying and mammals remained small creatures in dinosaurs’ ecological shadow. As we continue analyzing material from this remarkable site, each specimen enriches our understanding of ancient disease ecology while illuminating conserved principles of infection biology. The absence of healing in all six specimens particularly intrigues us. Did these individuals die during acute disease, or does this pattern suggest immune dysfunction across the population? Future isotopic analyses may reveal nutritional stress or environmental toxins that could have compromised immunity.
In studying these 80-million-year-old infections, we are not merely documenting extinct diseases. We are exploring how pathogens and hosts have coevolved across deep time, revealing that the microbial world’s impact on vertebrate populations transcends epochs, ecosystems, and even extinction events. The bones of these long-vanished giants remind us that microbes have always been, and remain, among evolution’s most powerful architects.
Prof. Dr. Tito Aureliano is a paleontologist at the Regional University of Cariri (URCA), Brazil. This research was supported by FUNCAP (FPD-0213-00295.01.01/23), with specimen curation by the Laboratory of Paleoecology and Paleoichnology at the Federal University of São Carlos.







No comments yet