Japanese encephalitis virus (JEV) is a flavivirus related to well-known viruses like West Nile, Dengue and Yellow fever and is the etiological agent of Japanese encephalitis (JE).
Whilst deemed a rare exotic tropical disease by the West, it is one of the leading causes of viral encephalitis globally, widespread across Asia-Pacific, endemic in 24 countries of south and southeast Asia. Each year it has been estimated that there are ~70,000 clinical cases of JE, impacting young children the most. This is because, generally, adults have acquired natural immunity from childhood infections. When an individual has been bitten by a mosquito, the virus replicates in Langerhans dendritic cells, which can then be carried to local lymph nodes for further replication. The virus amplifies, causing viremia, and when this occurs, the virus can cross the blood-brain barrier into the central nervous system. From there, encephalitis can occur. In these cases, around 30% lead to death, and up to 50% of survivors can have long-term neurological impacts from the central nervous system inflammation.
Japanese encephalitis viral evolution
JEV was first described in 1871 in Japan; however, it was not until over 60 years later that the virus was isolated in 1935. Its origin remains a mystery; however, researchers have alluded to its descendants from an African ancestral virus using phylogenetic comparisons with other flaviviruses. The most recent common ancestor of JEV is believed to have occurred 3255 years ago, predicted from Bayesian Markov chain Monte Carlo simulations conducted on whole genomic sequences of JEV genomes.
Five genotypes are distinguished by genome sequencing of the envelope gene (GI-GV). GI, GII, and GIII were first described in 1990, GIV in 1992 and GV in 2004; the genetic divergence of JEV genotypes descend from GV to GI. Until 1990, GIII was widespread throughout Asia until GI became more commonly detected. GI-GIII made up 98% of strains isolated between 1936 and 2009. GI is further subdivided into clades A and B based on geographical distribution. GIb currently dominates disease in seasonal epidemics of disease, and GIa, GII and GIV are associated with tropical endemics in children.
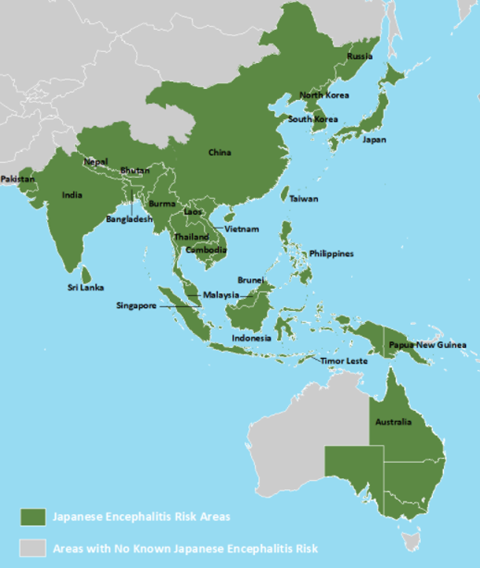
Disease transmission
JEV is an epizootic virus. The virus cycles from mosquitoes to vertebrate hosts. Hosts such as pigs are amplification hosts where high viremia is observed. The high viral replication level allows mosquitoes to be reinfected, sustaining viral transmission in endemic regions. Wading birds such as jacanas or herons are another key zoonotic host, as a ‘maintenance host’ (a population where pathogens remain present in the absence of transmission from other hosts maintaining the virus within the ecosystem). There are incidences of other vertebrates being infected with JEV, such as humans, horses and dogs, however, these are deemed dead-end hosts. These animals do not develop sufficient viremia for sustained viral transmission.
The transmission of JEV strongly correlates with environmental factors, including climate and land use. These environmental factors influence the mosquito vectors, impacting viral transmission. Studies have shown that incidences of JE are higher where pig and crop farming are close to human habitats, while disease prevalence drops in built-up urban areas. In addition, proximity to water bodies impacts the number of JE cases, a near-perfect breeding site for the vector, mosquitoes. Seasonal patterns play an essential role in the spread of JEV. Generally, transmission is high during the warmer months of the year, but during the rainy season, transmission intensifies due to the increase in mosquito vector circulation.

Treatment and prevention
Currently, there are no effective treatments against JE. The treatments focus on relieving symptoms and managing any downstream impact of the encephalitis, such as cognitive, psychiatric or neurologic symptoms.
There are a variety of vaccines available, with the first vaccine licensed in 1954 – a mouse brain-derived formalin-inactivated virus from BIRKEN. In 1968 an alternative vaccine was established using JEV cultivated from hamster kidney cells. Most recently, the IXIARO vaccine was developed, which is an attenuated strain vaccine of strain SA-14-14-2 grown in Vero cells. The live attenuated SA14-14-2 vaccine has become popular globally in endemic countries and for those travelling to endemic regions.
In the pursuit of effective vaccines, researchers have achieved high efficacies since the first licensed vaccine was available and have effectively reduced the burden of JE throughout Asia and the Pacific. In addition to vaccines, it is important not to forget traditional activities for disease prevention, such as avoiding mosquito bites using repellents, mosquito nets and wearing long sleeve clothing. These precautions prevent not only JEV transmission but also other mosquito-transmitted diseases which circulate in these regions.








No comments yet