Over the last 70 years, there has been an increase in the emergence of pathogens (mostly viral), from wildlife identified in humans. Several large-scale global disease outbreaks, including SARS-CoV-2, monkeypox, Middle East respiratory syndrome (MERS) and the Zika virus were all of animal origin.
Factors such as domestication of animals, encroachment into wildlife areas, global trade and travel, a growing population and urbanisation continue to increase human vulnerability to zoonotic spillover. The increasing incidence of zoonotic and vector-borne disease is also attributed to climate change which alters host-pathogen interactions. It has been estimated that a climate increase of 2° C by 2070 could result in over 15,000 new cross-species viral transmissions, which poses a significant threat to humans.
In today’s world, the spread of infectious diseases is fuelled by global travel and trade, and an increasing population with further opportunities for close contact. Failing to control localised outbreaks of disease makes the wider population vulnerable to global spread, as we are currently experiencing. However, limited surveillance and detection capacity due to poverty in endemic areas affects the prevention and control response to diseases such as monkeypox. Country-led poverty alleviation strategies are needed to establish effective surveillance and response systems and to improve healthcare services through both education and vaccine distribution.
Virology and characterisation
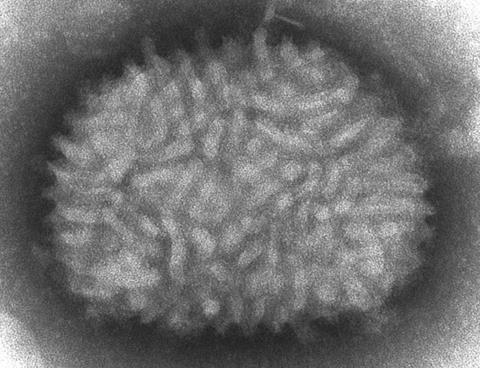
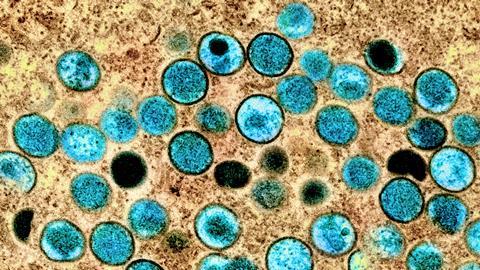
Human monkeypox infection is a zoonotic disease caused by monkeypox virus which belongs to the Orthopoxvirus genus of the Poxviridae family. Poxviruses are large, enveloped viruses with double-stranded DNA genomes which replicate in the cytoplasm of the host cell.
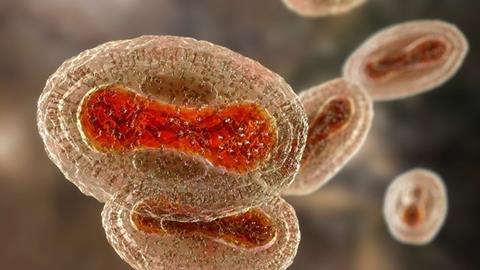
Monkeypox virus was first discovered in 1958 following an outbreak of pox-like disease among laboratory macaques in Copenhagen, Denmark. Despite its name, there is more evidence of infection in rodents such as Gambian pouched rats (Cricetomys gambianus), African dormice (Graphiurus spp.) and African ground squirrels (Xerus spp.). Although these small wild animals are capable of carrying the virus, further research is needed to identify specific hosts and their role in viral transmission within nature.
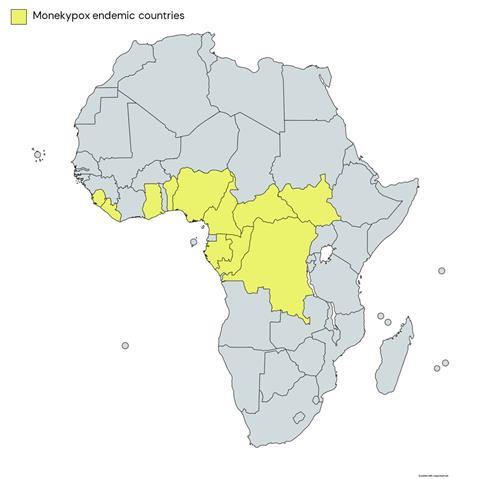
Since the first case of human monkeypox was reported in 1970 in the Democratic Republic of the Congo (DRC), formerly Zaire, it has spread to other regions of Africa (primarily West and Central). Human monkeypox is now endemic in 11 African countries: Benin, Cameroon, the Central African Republic, the DRC, Gabon, Côte d’Ivoire (Ivory Coast), Liberia, Nigeria, the Republic of the Congo, Sierra Leone, and South Sudan. Sporadic cases of monkeypox outside of Africa have also emerged in recent years linked to travel to endemic areas or through imported animals. In the current outbreak, however, there have been reported cases with no clear link to endemic areas.
Historically, monkeypox virus has been classified into two genetically distinct clades (variants), the Central African and West African clades. To avoid potential geographical stigma, the WHO recently reached a consensus to rename the clades as Clade I (formerly the Central African [Congo Basin] clade) and Clade II (formerly the West African clade). Clade II now consists of two subclades, IIa and IIb, with the latter referring to variants circulating in the ongoing 2022 outbreak. Clade I is associated with a higher mortality rate (around 10%) compared with Clade II (<1%).
Transmission
Human-to-human transmission of monkeypox virus occurs through close or direct contact with skin lesions, via large respiratory droplets, and possibly contaminated surfaces. There is no clear evidence of sexual transmission through seminal or vaginal fluids. Limited evidence suggests the presence of monkeypox DNA in seminal fluid during active infection, but this does not alone constitute evidence of sexual transmissibility. Kissing could be a risk if oral lesions are present. Vertical transmission (i.e. mother-to-child transmission) and foetal deaths have been reported but this was likely due to Clade I monkeypox virus.
In a recent study conducted in the UK, environmental samples were taken from isolation rooms of patients hospitalised with monkeypox. Viral DNA shed was detected on 93% (56/60) of surfaces and in 25% of air samples (5/20); 75% (3/4) of positive air samples were collected before and during a bedding change. Personal protective equipment (PPE) was also found to be contaminated following clinical contact and the changing of bedding. In addition to detecting viral DNA, the researchers found that some of the viruses from both the air- and surface samples were capable of infecting cells. It was acknowledged however that this does not necessarily mean that transmission resulting in infection would occur. Further research is required to assess the extent to which airborne transmission contributes to the spread of the virus. Furthermore, these findings highlight the importance of infection prevention and control measures within a healthcare setting through approaches such as the use of PPE and disinfectants, and negative pressure isolation rooms.
Symptoms
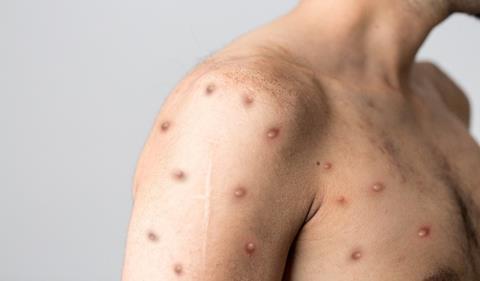
The incubation period (interval from infection to onset of symptoms) of monkeypox virus is generally 7–14 days but can range up to 21 days. In the early stages of infection, symptoms are non-specific and include fever, headache, chills, swollen lymph nodes and muscle aches. Around 1–5 days after fever onset, a rash first appears on the face before spreading to the extremities, including the palms of hands and soles of feet. It can also affect the mucous membranes in the mouth and genitalia. The rash progresses through four stages - macular, papular, vesicular, and pustular - before scabbing over and resolving over 2–3 weeks. Skin lesions are susceptible to secondary bacterial infection and thus require prompt identification and intervention.
During the current outbreak, atypical symptoms have been commonly reported including an absence or early onset of lesions, and localised anogenital lesions. Most cases up to now have been documented in gay, bisexual and other men who have sex with men (gbMSM). The high prevalence of genital lesions may be due to transmission of the virus via contact with infectious lesions during sexual intercourse. It is important to distinguish the disease from sexually transmitted diseases (STDs) with a similar clinical presentation including chancroid and syphilis.
Diagnosis of monkeypox disease can be confirmed by excluding other infections such as herpes simplex and varicella-zoster virus infection by polymerase chain reaction (PCR) of viral swabs from lesions, and by monkeypox virus PCR (now widely available in UK laboratories as well as at the UKHSA Rare and Imported Pathogens Laboratory, Porton Down). Infected individuals are advised to self-isolate at home and as much as possible avoid contact with others in the home. Isolation can end when i) they have not had a high temperature for over 72 hours, ii) all face, arm and hand lesions have scabbed and fallen off, iii) there have been no new lesions appearing for 48 hours, and iv) there are no mouth lesions.
Is monkeypox ‘under control’?
Until recently, monkeypox virus infection in humans was rarely reported outside of Central and Western Africa. On 6 May 2022, an outbreak of the disease was confirmed in the UK following a case from a British resident with travel links to Nigeria (an endemic country). Since then, the monkeypox outbreak has spiralled globally with most reported cases being from non-endemic countries.
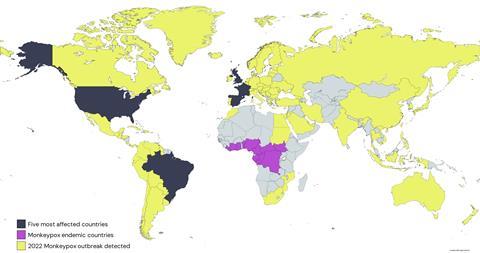
On 23 July, the World Health Organization (WHO) declared the escalating outbreak “a public health emergency of international concern.” Since the start of the year up to 13 October, the WHO reported 72,947 laboratory-confirmed cases of monkeypox and 28 deaths. The five most affected countries are the U.S. (n = 26,834), Brazil (n = 8,521), Spain (n = 7,239), France (n = 4,064), and the UK (n = 3,654). In the UK, 95% of cases have been reported in England, with London being the most affected.
Although clusters of infection remain, the overall rate appears to be declining and there does not appear to be a threat of a global pandemic. According to the UK Health Security Agency (UKHSA) and the European Centre for Disease Prevention and Control (ECDC), cases in the UK and EU/EEA peaked in July 2022 and have been steadily declining since. The WHO has also reported a decline in US cases since the peak in mid-August. However, this is not a time to relax, but rather to continue to enforce surveillance and control strategies.
Imvanex® (Bavarian Nordic, Hellerup, Denmark), a third-generation vaccine, contains an attenuated (weakened) Modified Vaccinia Ankara (MKA) strain and is approved in Europe and the UK, the U.S. (Jynneos®) and Canada (Imvamune®) for the prevention of smallpox. The European Medicines Agency (EMA) and the Food and Drug Administration (FDA) have permitted off-label use of the Imvanex® vaccine to support the ongoing fight against monkeypox virus. It is also considered safe for use in immunocompromised individuals. The public health response in the UK has focused on immunisation (using Imvanex®) for high-risk households and identified close contacts up to 14 days post-exposure.
The antiviral Tecovirimat inhibits p37, a conserved Orthopoxvirus protein, which prevents the formation and release of enveloped viruses. Tecovirimat is approved for use in both adults and children in the EU and the UK for the treatment of smallpox, monkeypox, cowpox, and vaccinia complications. Currently, in the UK, the antiviral drug is being offered to symptomatic patients with monkeypox disease who have been hospitalised with severe or complicated infections.
Should monkeypox be classified as a sexually transmitted disease?
There has been recent debate as to whether monkeypox disease should be classified as an STD given the high prevalence among gbMSM. It is important to note that many other viruses causing viraemia (presence in the bloodstream) can be found in semen with no direct evidence of sexual transmission. Because sexual contact is not the only mode of transmission, it is misleading to label monkeypox as an STD. Furthermore, viruses such as Zika virus have a clear route of sexual transmission yet are considered vector-borne diseases.
There are concerns that labelling monkeypox as an STD may introduce stigmatisation of those most at risk. It could also increase vulnerability among people with a misconception that this is the primary route of transmission. Effective risk communication and community-based engagement is crucial to raise awareness to aid in monkeypox disease prevention and control.
Next steps
Continued genomic surveillance is crucial to monitor the emergence of new variants of Orthopoxviruses (including monkeypox virus), to allow for an early response. Populations of wild rodents should also be monitored for infection to look for spillover into rodent reservoirs in nonendemic areas. Populations at risk should continue to be offered smallpox vaccination pre-exposure. Groups offered pre-exposure vaccine (although vaccine availability is limited) in the UK are gbMSM, sexual health clinic staff, staff in high consequence infectious disease units, and those decontaminating after monkeypox patients.
Risk factors should also be firmly established in order to review and update pre-exposure prophylaxis eligibility as needed.






No comments yet