ESKAPE is an acronym for: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species.
What are the ESKAPE pathogens?
The ESKAPE pathogens are a group of six bacteria which are both highly virulent and have increased levels of antimicrobial resistance (AMR), making them extremely challenging to treat. The ESKAPE pathogens are multidrug resistant (MDR) nosocomial pathogens which cause can cause life-threatening illnesses, often in immunocompromised patients. These pathogens are often isolated from hospital environments and contribute significantly to the burden of disease.
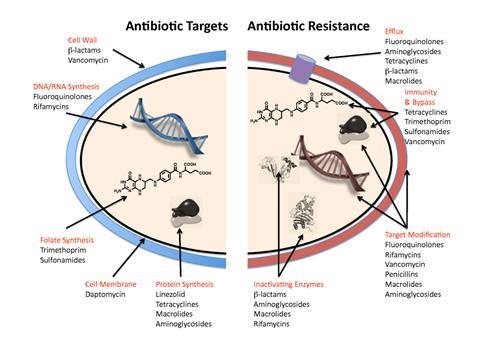
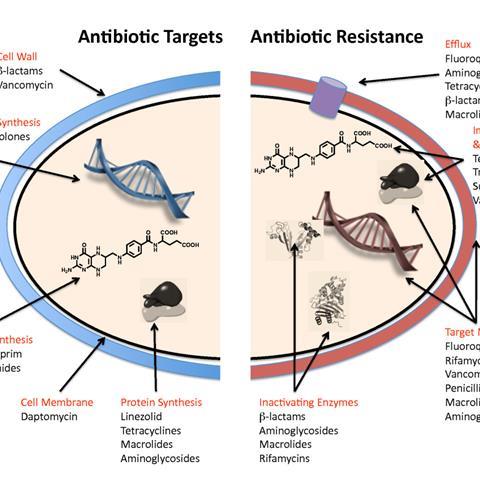
To evade the effects of antibiotics, the ESKAPE pathogens utilise a range of tactics including alteration of the bacterial target, antimicrobial degradation enzymes, expression of efflux pumps and biofilm production. The inappropriate and over use of antibiotics also drives AMR in the ESKAPE pathogens, selecting for the most resistant strains.
Enterococcus faecium
E. faecium is divided in to two subpopulations. The first is usually harmless and found in the human GI tract. However, the second subpopulation is responsible for hospital-acquired infections. Infection with E. faecium can cause illnesses such as urinary tract infections, bacteraemia and enterococcal infective endocarditis. The spread of E. faecium in hospital settings is mainly driven by high-contact points, shared equipment such as blood pressure cuffs and the hands of health care workers and visitors. The success of E. faecium in becoming a highly prolific hospital pathogen has been driven by the continual wide use of antimicrobials. Resistant E. faecium has shown resistance towards a wide-range of antibiotics, including low-dose penicillin and ampicillin, aminoglycosides, sulphonamides and cephalosporines.
Staphylococcus aureus
S. aureus can be found as part of the microbiota of healthy human skin and on mucus membranes, most commonly in the nasal passage. Although S. aureus does not cause disease in healthy individuals, it is an opportunistic pathogen which can cause a wide variety of clinical diseases including skin infections, urinary tract infections and toxic shock syndrome. These diseases can be acquired in the community and in the hospital environment. Treatment of infection is largely dependent on the type of infection and the drug-sensitivity of the infecting strain. Antibiotic-resistant strains such as Methicillin-Resistant Staphylococcus aureus (MRSA) can be resistant to multiple antibiotics, meaning that treating MRSA is increasingly complex.
Klebsiella pneumoniae
K. pneumoniae is a pathogen which can cause respiratory tract infections, pneumonia, and bloodstream infections. Infections with K. pneumoniae often occur in patients who require indwelling devices such as ventilators and are the leading cause of ventilator-associated pneumonia. The environment likely acts as a reservoir for human colonisation and infection with K. pneumoniae, being identified in water, sewage, soil and plants. K. pneumoniae strains can be categorised in to three main groups depending on its accessory genome: opportunistic, hypervirulent, and multidrug-resistant (MDR) groups. The MDR K. pneumoniae encode an enzyme called carbapenemsase, making these strains highly antibiotic resistant and increasingly challenging to treat.

Acinetobacter baumannii
A. baumannii is a successful hospital-acquired pathogen, responsible for opportunistic infections of the bloodstream, skin, urinary tract, and other soft tissues. Most A. baumannii infections occur in patients who have prolonged hospital stays, have indwelling medical devices or are in intensive care units. MDR is severe problem when treating A. baumannii infection as it is resistant to many classes of antibiotics, including carbapenems. Research shows that the bacterium can rapidly develop resistance mechanisms and degrade antibiotics to evade the effects of the antibiotics. MDR A. baumannii previously gained the nickname ‘Iraqibacter’ as there was a sharp increase in reports of infection in soldiers in Iraq and Afghanistan in 2003. It is thought that MDR A. baumannii was then able to spread to civilian hospitals through infected soldiers.

Pseudomonas aeruginosa
P. aeruginosa is an opportunistic pathogen which can be found in the environment, mainly in soil and ground water. P. aeruginosa rarely causes disease in healthy people and is more likely to cause disease in individuals who are immunocompromised. Infection with P. aeruginosa can occur by contact with contaminated water or soil. In the hospital environment, P. aeruginosa is spread through high-contact surface areas, contaminated surfaces and medical equipment. P. aeruginosa can cause a variety of illnesses once it has entered the body and commonly infects the lungs of patients with cystic fibrosis. The prevalence of MDR P. aeruginosa strains means that treatment of the infection is becoming increasingly challenging, leaving limited treatment options available.
Enterobacter species
Infections with Enterobacter spp. can cause various diseases, including meningitis, septicaemia, wound infections, and pneumonia. Enterobacter spp. have environmental reservoirs in water, soil and occasionally in food sources if contaminated water is used for crop maintenance. Enterobacter spp. are resistant to multiple antibiotics and express various enzymes to degrade antibiotics including carbapenemases and extended-spectrum beta-lactamases.

How can we reduce the risk in hospitals of infection with an ESKAPE pathogen?
Hand-hygiene of patients and visitors is an essential step in tackling any hospital acquired infection. This reduces the overall chance of getting sick and further reduces the risk of transmitting any pathogens on the hands to surfaces or medical equipment. The routine cleaning of medical equipment and surfaces is also key, although it is important to consider the types of cleaning equipment used as antimicrobial sprays may drive further resistance.








No comments yet