A team at Queen’s University Belfast have developed a non-invasive sensor that changes colour when a wound becomes infected, alerting healthcare staff hours before symptoms become visible to the eye.
Dr Erin Magee, a Research Fellow in Applied Microbiology International president Professor Brendan Gilmore’s lab, outlined the development of the 3D printed optical sensor to delegates at the recent Letters in Applied Microbiology ECS Research Symposium at Riddel Hall in Belfast.

The work was the result of a collaboration with Prof Andrew Mills at the School of Chemistry, and offered a method of non-invasive monitoring of wound infection to address the current crisis in wound management.
Chronic wounds
Chronic wounds can be defined as wounds which fail to heal within three months, and affect approximately 2% of the worldwide population, incurring healthcare costs of £5.6 billion in the UK alone, Dr Magee told delegates.
“Owing to an ageing population and a substantial rise in predisposing factors such as obesity, diabetes and cardiovascular disease, chronic wounds have been described as a silent epidemic, the full financial impact of which is immeasurable,” she said.
“Key to their persistence is the formation of microbial biofilms, which are accounted for in nearly 80% of all non-healing wounds.
Dressing changes
Current wound care practices for monitoring infection development and treatment rely on regular dressing changes to visually inspect the wound for signs of deterioration or improvement.
“Dressing changes cause great discomfort to the patient, increase the likelihood of infection through repeated exposure to the surrounding environment, and disturb the reparative process of healing wounds,” Dr Magee said.
The team tackled these problems by developing a novel 3D printed sensor to offer 24/7 non-invasive monitoring of wound infection to overcome the shortcomings of current wound care and provide an early warning to infection development and unsuccessful treatment.
Monitoring headspace
The sensor monitors the headspace that exists between the wound and dressing for small increases in carbon dioxide, which is ubiquitously produced from aerobic microorganisms to provide an all-encompassing marker of infection.
“Preliminary work involved testing several candidate CO2-sensitive dyes for response time, colour change and colour intensity to common wound pathogens. Xylenol blue proved the strongest candidate, with a stark colour change of blue to yellow,” Dr Magee said.
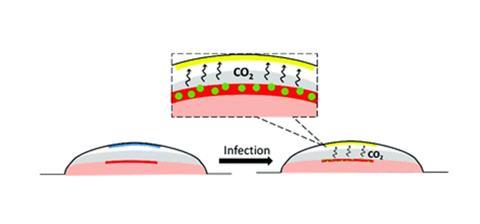
“Following selection of xylenol blue dye, the formulation was 3D printed to form an indicator square and tested against a range of common wound pathogens in a newly developed ex vivo porcine skin wound dressing model.
“Film colour change in response to inoculation was monitored by taking hourly photographs of the film followed by colour analysis. The time taken for the sensor to change colour was linearly correlated to peak metabolic activity using isothermal calorimetry, and a wound bioburden of 106 CFU/g, which is considered the threshold of clinical infection and progression to a non-healing state.
Early warning system
“In addition, the sensor was shown to change colour before robust biofilm formation by Pseudomonas aeruginosa, which demonstrates the sensor’s potential as an early-warning indicator of biofilm development.
“These results were very encouraging and demonstrated the potential of the indicator film to detect early-stage infection in wounds. Current practices for monitoring wound infection treatment have several downfalls with limited alternatives; hence, the potential of this sensor for non-invasive therapeutic monitoring was realised and exploited in the final body of work.”
Biofilms grown
For this piece of work, biofilms of P. aeruginosa were grown on porcine skin explants and subsequently treated with an antibiotic agent. The treated explants were then packaged in the wound dressing model and the sensor response monitored.
The bioburden of the wound fluid surrounding the treated explant was determined and compared with the sensor response.
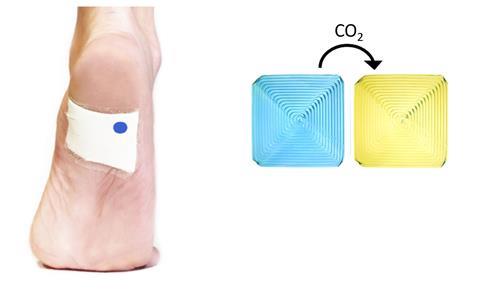
Unsuccessful treatment with sublethal concentrations of ciprofloxacin or gentamicin was reflected by the sensor changing colour from blue to yellow, indicating the presence of rapidly dividing and metabolically active P. aeruginosa.
Accurate indication
In contrast, sensors monitoring successfully treated wound biofilm remained blue, indicating complete eradication of P. aeruginosa.
A greater log-fold reduction in wound bioburden following treatment was complemented by a longer sensor response time, confirming that the sensor could be used to accurately monitor treatment efficacy in a non-invasive manner.
Implementation of the sensor has the potential to aid the wound care revolution by meeting many of the desirable and essential criteria for better infection diagnostics, Dr Magee said.
Versatile sensor
“The sensor is biocompatible, sensitive, inexpensive, amenable to scalable production and can be incorporated into any dressing type. In addition, the colour change is easily interpreted with the naked eye and does not require complicated post-processing,” she said.
“Patients can rely on this sensor to monitor wound condition outside the healthcare setting, which provides peace of mind and encourages one of the new practice proposals for better wound care – patient self-management.
“The sensor responds to a wide variety of common wound pathogens, which proves its suitability for monitoring infection development in an environment that is known to be microbially diverse.
Precise monitoring
“The sensor can provide precise monitoring of treatment efficacy, enabling undisturbed healing of successfully treated wounds whilst also alerting healthcare providers to intervene when treatment is failing.
“The next step of this research is to test the sensor in a more realistic model of wound infection involving live test subjects. We used porcine skin for our preliminary studies because it closely mimics human skin - however, this work is now ready to progress on to in vivo testing.”
Part of this study was published in the journal Chemical Communications last year.
This work was led by Professor Brendan Gilmore (School of Pharmacy, QUB) and Professor Andrew Mills (School of Chemistry, QUB). I worked alongside Dr Dili Yusuf (Research fellow, School of Chemistry, QUB). Work funded by Department for the Economy and EPSRC.
Topics
- Andrew Mills
- Applied Microbiology International
- Bacteria
- Biofilms
- Brendan Gilmore
- Clinical & Diagnostics
- Early Career Research
- Erin Magee
- Events
- Innovation News
- Letters in Applied Microbiology ECS Research Symposium
- One Health
- Pseudomonas aeruginosa
- Queen’s University Belfast
- sensor
- UK & Rest of Europe
- wound management






No comments yet