The first all-oral treatment for Trypanosoma brucei (T.b.) rhodesiense sleeping sickness, an acute form of the disease, is now available free of charge to patients in specialized treatment centres in Ethiopia, Malawi, Tanzania, Zambia, and Zimbabwe.
Ministries of Health in several African countries have approved the use of Fexinidazole Winthrop for T.b. rhodesiense sleeping sickness treatment.
”Until now, the only treatment for the advanced stage of the disease involved a toxic intravenous drug that required hospitalization. Today, with this breakthrough, we have a safe and simple oral treatment that can be taken at home with minimal observation, revolutionizing care for patients. The authorization of Fexinidazole Winthrop in Malawi and several other African countries is a testament to the dedication and hard work of African doctors, clinicians, healthcare staff, and communities who contributed to its development,” said Dr Westain Nyirenda, principal investigator of the clinical trials for Fexinidazole Winthrop in Malawi.
READ MORE: Atomic imaging and AI offer new insights into motion of parasite behind sleeping sickness
READ MORE: Novel point of attack to combat dangerous tropical diseases
The clinical trials that led to Fexinidazole Winthrop’s approval were sponsored by the not-for-profit medical research organization Drugs for Neglected Diseases initiative (DNDi).
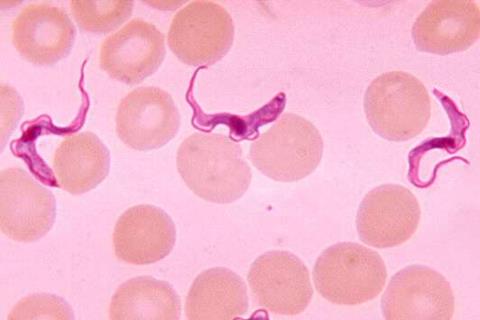
Sleeping sickness, or human African trypanosomiasis (HAT), is a parasitic disease transmitted by the bite of tsetse flies. It causes neuropsychiatric symptoms, including a debilitating disruption of sleep patterns and ultimately coma and death. It is almost always fatal if not treated. The T.b. rhodesiense form of the disease, which occurs in East and Southern Africa, progresses more rapidly than the T.b. gambiense form, which is endemic to Western and Central Africa.
Innovative partnership
Fexinidazole Winthrop was developed through an innovative partnership that brought together Sanofi, DNDi, national sleeping programmes, and local communities. The European Medicines Agency issued a positive scientific opinion for treatment of T.b. gambiense with Fexinidazole Winthrop in 2018. For T.b. rhodesiense sleeping sickness, DNDi led a Phase II/III clinical trial in Malawi and Uganda supported by a consortium of partners known as HAT-r-ACC that showed the treatment is a better alternative to existing drugs.
The results led the European Medicines Agency to issue a positive scientific opinion in December 2023. Following this decision, a regulatory approval was given in May 2025 by the regulatory authorities of the Democratic Republic of the Congo and subsequently, Malawi approved its use in December 2024. In June 2024, the World Health Organization (WHO) included it as the first-choice treatment for rhodesiense sleeping sickness in its treatment guidelines. Since the beginning of 2025, importation and distribution of the drug have been approved in the five African countries listed above and received shipments from WHO. Several patients have already received this life-saving treatment in Malawi, Zambia, and Zimbabwe.
Deadly outbreaks
Deadly outbreaks of rhodesiense sleeping sickness still occur, most recently in Malawi from 2019 to 2021. A localized rhodesiense outbreak in Ethiopia in 2022 – the first in 30 years – has been linked to climate and environmental changes that bring humans and animals such as cattle in closer proximity to the tsetse flies that carry the disease. Safari tourists from Europe and the US visiting the region have also fallen ill with this strain of sleeping sickness and have received Fexinidazole Winthrop under compassionate use protocols in Austria, Denmark, Poland, and the United States.
”With climate and environmental changes increasing the risk of future rhodesiense outbreaks, we are now prepared to meet these challenges head-on with all-oral treatments, which will save lives and ease the burden on our healthcare systems in Africa,” said Dr Junior Matangila, Head of DNDi’s sleeping sickness programme. ”This is important, as it will be difficult to interrupt transmission of the rhodesiense form of sleeping sickness because it has an animal reservoir. On the other hand, as the reservoir of gambiense sleeping sickness is essentially human, interruption of transmission is an attainable goal. So far, eight countries have eliminated gambiense sleeping sickness as a public health problem, the latest being Guinea earlier this year.”
All-oral treatment
Fexinidazole Winthrop is recommended for adults and children aged six years or older and weighing at least 20 kg who have been diagnosed with either first-stage (haemolymphatic) or second-stage (meningoencephalitic) rhodesiense sleeping sickness in addition to the treatment of gambiense sleeping sickness approved in 2018. The all-oral treatment is donated to the WHO by Foundation S, Sanofi’s philanthropic organization, and delivered to Africa by the Médecins Sans Frontières Logistique supply centre.
”We are thrilled to see the access and use of Fexinidazole Winthrop as the first fully oral treatment to treat rhodesiense sleeping sickness in Africa. This milestone underscores Sanofi’s unwavering long-term commitment to addressing neglected tropical diseases challenges and improving patient outcomes. Through Foundation S, we are dedicated to providing innovative treatments to those who need them most, ensuring that no patient is left behind. This approval is a testament to the power of collaboration and the impact we can achieve together, contributing further to eliminate sleeping sickness,” said Philippe Neau, Head of the Neglected Tropical Diseases (NTDs) Programme at Foundation S, the Sanofi collective.
The DNDi clinical trial for T.b. rhodesiense sleeping sickness was conducted by the HAT-r-ACC Consortium, with funding from the European and Developing Countries Clinical Trials Partnership (EDCTP2) programme supported by the European Union (through the grant RIA2017NCT-1846); Fundação para a Ciência e a Tecnologia, Portugal; the Swiss Agency for Development and Cooperation (SDC); Médecins Sans Frontières; UK International Development; and other private foundations and individuals.
Topics
- Disease Treatment & Prevention
- Drugs for Neglected Diseases initiative
- Fexinidazole Winthrop
- Foundation S
- Infection Prevention & Control
- Junior Matangila
- Middle East & Africa
- One Health
- Parasites
- People News
- Pharmaceutical Microbiology
- Philippe Neau
- sleeping sickness
- Trypanosoma brucei (T.b.) rhodesiense
- UK & Rest of Europe
- Westain Nyirenda






No comments yet