Infections caused by Pseudomonas aeruginosa biofilm significantly endanger human health worldwide. Biofilms are closely associated with antibiotic resistance because biofilms significantly undermine the efficacy of antibiotics.
A novel ultrasound-launched targeted nanoparticle was developed to universally destroy biofilm, target bacteria, deliver antibiotics, and efficiently kill bacteria via ultrasonic cavitation and antibacterial sonodynamic therapy.
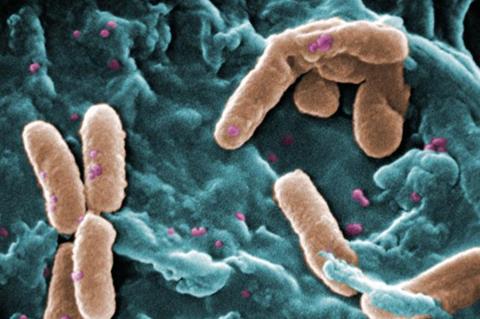
The nanoparticle consisted of poly (lactic-co-glycolic acid) loading ciprofloxacin and perfluoropentane with a bacteria-targeted antibody installed on the nanoparticle for binding to specific bacteria. The nanoparticle exhibited a sensitive response to ultrasound and the rapid liquid-gas phase transition of perfluoropentane resulted in a cavitation effect that destroyed the extracellular polymeric substances of the biofilm and allowed deep penetration of the antibiotics.
In addition, ciprofloxacin induced additional reactive oxygen species production under ultrasound stimulation, leading to an enhanced bactericidal effect and potent anti-infective activity in vivo. This study, published in BIO Integration, presents an effective strategy to tackle the extracellular polymeric substance barriers for overcoming antibiotic resistance and removing a biofilm.





No comments yet