The International Vaccine Institute (IVI) and EuBiologics Co Ltd. have announced the licensure for export on December 19, 2023 by the Korean Ministry of Food and Drug Safety (KMFDS) of Euvichol-S, a simplified formulation of the oral cholera vaccine (OCV) Euvichol-Plus® that is prequalified by the World Health Organization.
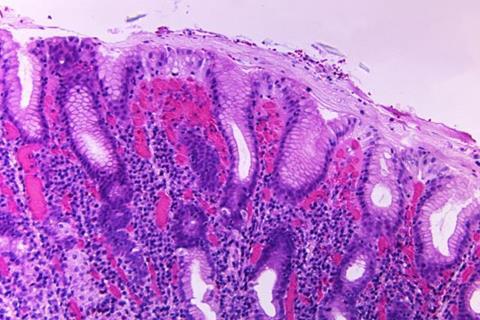
The licensure of Euvichol-S is the culmination of a comprehensive phase 3 clinical trial conducted by IVI and paves the way for a potential solution to the critical shortage of OCV worldwide.
IVI’s commitment to addressing the global cholera crisis has led to the development of Euvichol-S, aimed at ensuring an adequate and cost-effective supply of the life-saving vaccine. The ongoing global shortage of OCV has hindered the deployment of effective cholera control efforts, exacerbating the impact of unprecedented cholera outbreaks in 2022 and 2023.
Vaccine shortage
Dr. Julia Lynch, Director of IVI’s Cholera Program, said: “Current vaccine production capacity at 33 million doses this year falls notably short in meeting current and forecasted demand.
“The simplified formulation is expected to increase vaccine production capacity at lower production cost, potentially increasing global supply toward the requirement estimated by Gavi, the Vaccine Alliance.”
Reformulation process
In 2019, IVI received support from the Bill & Melinda Gates Foundation to reformulate Euvichol-Plus, with the potential to reduce production costs by 20% and increase production capacity by 38%. IVI reformulated Euvichol-Plus by reducing components from five to two and the inactivation process from two to one.
The Phase 3 clinical trial comparing the lower-cost formulation, Euvichol-S, to Shanchol was successfully completed in Nepal in October 2022 to demonstrate the non-inferiority of Euvichol-S compared to ShancholTM, laying the foundation for regulatory approvals. An application for licensure was submitted to KMFDS in March 2023, with a concurrent review for pre-qualification by the WHO.
The trial, encompassing 2530 participants, confirmed non-inferior seroconversion rates for Euvichol-S against V. cholerae O1 Inaba and Ogawa compared to ShancholTM. With a robust safety profile, Euvichol-S emerges as a promising solution to bridge the gap between limited OCV supply and rising demand.
Safety profile
Dr. Katrina Rok Song, IVI’s project lead of the trial, said: “The clinical trial results demonstrated immune non-inferiority and safety of the Euvichol-S vaccine compared to ShancholTM. The safety profile of Euvichol-S was comparable to that established for ShancholTM, where millions of doses have been administered since its approval in 2009.”
With the addition of Euvichol-S, EuBiologics is poised to ramp up its OCV production to 52 million doses including more than 15 million doses of Euvichol-S in 2024, while boosting its overall capability to produce OCV materials to up to 90 million doses annually. This boost in production is expected to alleviate the chronic shortage of low-cost OCV and contribute substantially to global cholera prevention efforts.
Manufacturing preparation
Dr. Yeongok Baik, CEO of EuBiologics, said: “We have already completed preparation for Euvichol-S manufacturing at our GMP facility, and will be able to supply more than 15 million doses of this vaccine in 2024. This change aligns with global efforts, such as WHO’s ‘Ending Cholera – A Global Roadmap to 2030,’ targeting a 90% reduction in cholera deaths through preventive OCV campaigns.”
The roadmap proposed by the Global Task Force on Cholera Control of WHO requires an estimated 670 million doses of OCV for preemptive use in high‑risk areas in order to optimize prevention of the disease.
Dr. Jerome Kim, Director General of IVI, said: “The need for cholera vaccine is urgent because of the large and unanticipated number of cholera outbreaks, so the licensure of Euvichol-S marks a significant step toward addressing the critical shortage of cholera vaccine globally. As the world grapples with the challenges of cholera, IVI remains committed to advancing innovative solutions to save lives and protect people in collaboration with partners worldwide.”
Topics
- Asia & Oceania
- Bacteria
- cholera
- Clean Water
- EuBiologics Co Ltd.
- Euvichol-Plus®
- Euvichol-S
- Industry News
- International Vaccine Institute
- Jerome Kim
- Julia Lynch
- Katrina Rok Song
- Korean Ministry of Food and Drug Safety
- O1 Inaba
- Ogawa
- One Health
- Sample preparation, filtration, detection and treatment techniques for water-borne pathogens
- Vaccinology
- Vibrio cholerae
- Yeongok Baik





No comments yet