All Industry News articles
-
 News
NewsCARB-X to support development of typhoid fever diagnostic from Chembio
CARB-X is awarding US$1.8 million to Chembio Diagnostic Systems, Inc. to develop a rapid point-of-care test for the detection of Immunoglobulin A (IgA) antibodies to diagnose acute infection of Salmonella enterica serovar Typhi.
-
 News
NewsPIXL Max: Automated colony picker breaks records for speed and accuracy
With the latest innovation from the team at Singer Instruments, a new standard for speed and repeatability in microbial colony pickers has been achieved through the integration of cutting edge AI technologies.
-
 News
NewsNexaBiome accelerates development of novel diabetic foot treatment with Scottish Enterprise funding
UK biotechnology company NexaBiome Life Sciences Ltd has received continued funding from Scotland’s national economic development agency, Scottish Enterprise, to accelerate its breakthrough bacteriophage technology for the treatment of diabetic foot infections (DFIs).
-
 News
NewsUK Centre for Mould Safety launches national training academy
The UK Centre for Mould Safety (UKCMS) National Training Academy has today opened its doors to upskill and improve competence, consistency and safety across all industries that serve homes and buildings, in a drive to support public health outcomes.
-
 News
NewsValneva provides update on Chikungunya vaccine IXCHIQ®
Valneva SE has announced that the company has decided to voluntarily withdraw the biologics license application (BLA) and Investigational New Drug (IND) application for its chikungunya vaccine, IXCHIQ® , in the United States, following suspension of the license by the FDA.
-
 News
NewsVaccine against foot-and-mouth disease could deliver $1.3 billion a year in global livestock benefits
A new foot-and-mouth disease vaccine is projected to deliver over $1.3 billion in annual benefits and transform global livestock resilience.
-
 News
NewsCFA publishes timely new industry-led UK Ready to Eat Foods Safety and Shelf Life Guidance
New industry-led good practice guidance for manufacturers and retailers of certain ready to eat (RTE) foods will be published on 12 January 2026 to help Food Business Operators (FBOs), Competent Authorities (CAs) and enforcement officers manage the risk posed by Listeria monocytogenes in those products.
-
 News
NewsStrategic advancement of second-generation fungal vaccine VXV-01 through Phase 1 trials
The Lundquist Institute (TLI) and its start-up company Vitalex Biosciences (Vitalex) have announced that the second-generation fungal vaccine candidate known as VXV‑01 is poised to move forward in development up to and including Phase 1 clinical evaluation.
-
 News
NewsCARB-X to support investigation of Exhalon’s breath-based lower respiratory tract infection testing platform
CARB-X has awarded Exhalon US$1M in seed funding to evaluate whether exhaled breath can be used as a non-invasive sample type to aid in the rapid and accurate diagnosis of lower-respiratory tract infections (LRTIs).
-
 News
NewsAFYREN and ESSE Skincare begin partnership to offer 100% natural cosmetic solutions with enhanced skincare performance
AFYREN, a greentech company offering manufacturers biobased, low-carbon ingredients through a unique fermentation technology, and South Africa-based Esse Skincare, a leader in microbiome skincare science, are partnering to introduce the world’s first bio-based propionic acid for the skincare industry.
-
 Careers
CareersThe Future is Fungi 2025: award-winner Michroma’s mission to harness fungi for clean food dyes and flavors
Winner of The Future is Fungi Award 2025, US and Argentina-based foodtech startup Michroma is replacing petrochemical coloring with fungibased natural ingredients, launching one of the world’s leading sustainable platforms for food flavors and colors. Here’s its story.
-
 News
NewsApriori Bio and A*STAR Infectious Diseases Labs Announce strategic partnership to advance next generation influenza vaccines
Apriori Bio and the Agency for Science, Technology and Research Infectious Diseases Labs (A*STAR IDL) announced a strategic research partnership to co-develop and evaluate next generation self-amplifying RNA (saRNA) vaccines targeting seasonal and pandemic influenza.
-
 News
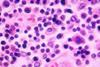
NewsTyphoid conjugate vaccine demonstrates strong safety and immunogenicity: Results from Phase 3 study
PATH and EuBiologics Co., LTD have announced Phase 3 results from a clinical trial of a typhoid conjugate vaccine (TCV), EuTYPH-C Inj.® Multi-dose. EuTYPH-C Inj.® Source: CDC/ Armed Forces Institute of Pathology, Charles N. Farmer This photomicrograph reveals some of the histopathology exhibited in a lymph node tissue ...
-
 News
NewsCARBIOS and Wankai New Materials to build PET biorecycling plant in China
CARBIOS and Wankai New Materials, a subsidiary of Zhink Group, are committed to the large-scale deployment of CARBIOS’ PET biorecycling technology in Asia, with the first step being the construction of a PET biorecycling plant in China.
-
 News
NewsUK’s failure to retain and scale science and technology causing economy to bleed out, warns Lords Committee
The UK’s failure to retain and scale its science and technology companies has now reached crisis point and is causing the UK economy to bleed out, warns a damning report.
-
 News
NewsBruker announces FDA Clearance of Claims 7 and 8 for the MALDI Biotyper® CA System
Bruker has announced FDA clearance of Claim 7 and Claim 8 for its MALDI Biotyper® CA System, marking a significant advancement in clinical microbial identification capabilities.
-
 News
NewsPioneering gut health testing with a simple finger prick
Zinzino, the Scandinavian health and wellness company, has announced the launch of its innovative Gut Health Test. With a simple at-home finger prick, this is the first commercial test to measure what gut bacteria produce and how the body responds.
-
 News
NewsCaldic and AmphiStar join forces to transform personal care market in Europe with upcycled microbial biosurfactants
Global distributor Caldic and Belgian biotech innovator AmphiStar have announced an exclusive partnership to distribute and promote AmphiStar’s 100% upcycled microbial biosurfactants for personal care applications across Europe.
-
 News
News Bioinsecticide start-up BugBiome focuses on lead product development with move to Norwich Research Park
BugBiome, the agri-tech innovator developing new bioinsecticides from crop-associated microbes, has relocated to Norwich Research Park as it focuses on moving its lead aphicide into field trials in 2026.
-
 News
NewsMolecular Sustainable Solutions receives investment from BeAble Capital to boost disinfection and sterilization methods
Molecular Sustainable Solutions, a spin-off from the Universitat Jaume I of Castelló (UJI), secures €186,000 investment from BeAble Capital, a leading Science Equity fund specializing in disruptive scientific technologies.
