The Drugs for Neglected Diseases initiative (DNDi) and Sanofi have announced treatment success rates of up to 95% from a Phase II/III study investigating the safety and efficacy of single-dose acoziborole, a potentially transformative investigational treatment for sleeping sickness.
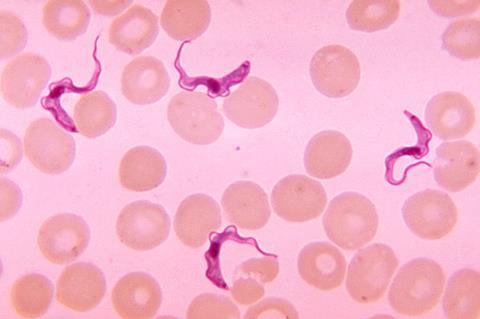
The clinical trial was led by DNDi and its partners in the Democratic Republic of the Congo (DRC) and Guinea, and is published in The Lancet Infectious Diseases medical journal.
”Sleeping sickness is a nightmare disease that affects patients in some of the most remote settings in West and Central Africa, where distance from hospital can be measured in days,” said Dr Victor Kande, former Neglected Tropical Diseases Expert Advisor at the Ministry of Health of the DRC, principal investigator of the trial, and lead author of the Lancet article.
‘We are now on the cusp of a potential treatment that can be given in one day, in a single dose of three pills – this would be a revolution for doctors and communities.’
Transmitted by the bite of an infected tsetse fly, sleeping sickness is fatal without treatment. In the early stage of the disease, people suffer from headaches or fever. In the late stage, the parasite crosses the blood-brain barrier and invades the central nervous system, causing neuropsychiatric symptoms such as sleep disruption, confusion, lethargy, and convulsions – and ultimately, death.
Improving treatments
Treatment for sleeping sickness has radically improved in the last decade, thanks to the successes of a partnership bringing together DNDi, Sanofi, the national sleeping sickness control programmes of the DRC and Guinea, the World Health Organization (WHO), Médecins Sans Frontières (MSF), and other partners.
The number of reported cases of sleeping sickness has fallen sharply in the last 20 years, from almost 40,000 reported cases in 1998 (with estimates of over 300,000 undiagnosed cases) to less than 1,000 in 2020. Though an encouraging trend, it should not be a reason for complacency, as the disease is known to resurge in the form of devastating outbreaks.
”By simplifying the treatment paradigm, acoziborole would be an innovation that enables a sustainable response to sleeping sickness for health systems. With these new data, we have hope that we may be able to finally eliminate the disease, once and for all, by opening the door to a ‘screen-and-treat’ approach at the village level,” said Dr Antoine Tarral, Head of the sleeping sickness programme at DNDi and co-author of the paper.
”Acoziborole has the potential to have a major impact on sleeping sickness treatment,” said Dr Wilfried Mutombo Kalonji, DNDi Clinical Project Leader and Medical Manager, and co-author of the paper.
”This is a complicated disease, with patients living in very remote and unstable areas. We need simple approaches. We now have the promise of single-dose, oral treatment that doesn’t need to be taken with food, removes the need for hospitalization, removes the need for complex or invasive diagnosis or disease staging, and requires minimal training. In many ways, it doesn’t get any simpler than this.”
The WHO Neglected Tropical Diseases Roadmap strategy sets a goal of interrupting transmission of the gambiense strain of human African trypanosomiasis (g-HAT), the scientific name of the disease, by 2030. Acoziborole could open the possibility of a simple ‘screen-and-treat’ approach, whereby any person whose rapid pinprick blood test suggests they might be infected could be treated with acoziborole, without the need for more complex parasitological confirmation or hospitalization.
The study
Between 2016 and 2019, DNDi and its partners led an open-label, Phase II/III study to assess the safety and efficacy of acoziborole in patients with early- and late-stage g-HAT. 208 patients were recruited at 10 hospitals in the DRC and Guinea.
The 18-month treatment success rate for acoziborole was 95% in late-stage g-HAT patients, corresponding to the best results from studies with existing treatments (94%). In addition, 100% of the 41 patients with early-stage g-HAT were considered as treatment successes at all timepoints. The study shows that acoziborole has a favourable safety profile, with no significant drug-related safety signals reported.
These pivotal results will form the basis of Sanofi’s dossier submission to the European Medicines Agency (EMA) and represent another milestone in the quest to eliminate sleeping sickness. Upon the EMA’s positive opinion and local approval, Sanofi will donate acoziborole to the WHO through its philanthropic organization, Foundation S – The Sanofi Collective. Acoziborole is currently under clinical investigation and its safety and efficacy have not been evaluated by any regulatory authority.
”Sanofi is a long-standing partner in the fight against sleeping sickness alongside DNDi and the World Health Organization,” says Dietmar Berger, MD, PhD, Head of Development and Chief Medical Officer, Sanofi.
”We are leveraging Sanofi’s unique expertise in developing, registering and producing new, innovative medicines to address areas of significant unmet need and in this case, support the World Health Organization’s goal to eliminate sleeping sickness in humans by 2030.”
”Many sleeping sickness patients live in remote villages in the mangrove swamps along the coast of Guinea – even though we have good treatment options now, we still need to send people who test positive with a sleeping sickness rapid test from their village to a confirmatory health centre,” said Dr Mariame Camara, principal investigator of the study at the HAT Treatment Centre in Dubreka, Guinea and co-author of the Lancet article.
”Acoziborole could potentially become the first treatment that would allow doctors to treat people on the spot, once they receive a positive rapid test in their village.”
Topics
- Acoziborole
- Democratic Republic of the Congo
- Disease Treatment & Prevention
- DRC
- Drugs for Neglected Diseases initiative
- European Medicines Agency
- g-HAT
- Guinea
- Industry News
- Infectious Disease
- Innovation News
- Middle East & Africa
- One Health
- Parasites
- Parasitology
- Sanofi
- sleeping sickness
- trypanosomiasis
- tsetse fly
- WHO Neglected Tropical Diseases Roadmap






No comments yet