The gut microbiome is so useful to human digestion and health that it is often called an extra digestive organ. This vast collection of bacteria and other microorganisms in the intestine helps us break down foods and produce nutrients or other metabolites that impact human health in a myriad of ways.
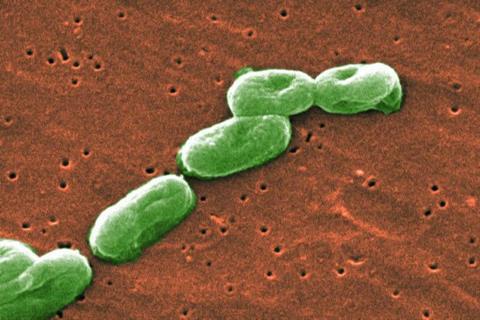
New research from the University of Chicago shows that some groups of these microbial helpers are amazingly resourceful too, with a large repertoire of genes that help them generate energy for themselves and potentially influence human health as well.
The paper, published in Nature Microbiology, identified 22 metabolites that three distantly related families of gut bacteria use as alternatives to oxygen for respiration in the anaerobic environment of the gut. These bacteria also have up to hundreds of copies of genes for producing the enzymes that process these alternate metabolites – many more than have been measured in bacteria that live outside the gut. These results suggest that anaerobic gut bacteria may have the ability to produce energy from hundreds of other compounds as well.
Peculiar metabolisms
“These are examples of some of the peculiar metabolisms that act on all these different metabolites produced by the gut microbiome,” said Sam Light, PhD, Neubauer Family Assistant Professor of Microbiology at UChicago and senior author of the study. “This is interesting because one of the main ways the microbiome impacts our health is by making or modifying these small molecules that can then enter our bloodstream and act like drugs.”
At the organism level, we typically think of respiration as the process of breathing in oxygen. At the cellular level, respiration describes an energy-generating biochemical process. Most cells use oxygen for respiration, but in anaerobic environments like the inside of the intestine, cells have evolved to use other molecules.
Cells possess two main types of metabolism to produce energy: fermentation and respiration. In fermentation, the cell breaks down molecules to generate energy directly. Respiration involves two molecules: an electron donor and an electron acceptor. A classic example of this process uses glucose as a donor and oxygen as the acceptor. The cells break down the glucose by shuttling extracted electrons through a series of steps before their final transfer to an oxygen molecule. This prompts the cell to generate ATP, or adenosine triphosphate: the basic source of energy for use and storage at the cellular level.
Database of genomes
Most of the microbes living in the gut use fermentation, but there are also several known types of bacteria with respiratory metabolisms, including those that use carbon dioxide and sulfate electron acceptors. For the new study, Light and his colleagues analyzed a database of more than 1,500 genomes from a database of human gut bacteria. They saw a surprising distribution of genes that produce reductases, which are enzymes that use different respiratory electron acceptors.
While most of the genomes encode just a few reductases, a small subset encodes more than 30 different ones. These bacteria weren’t closely related; they came from three distinct and distantly related families (Burkholderiaceae, Eggerthellaceae, and Erysipelotrichaceae) separated by hundreds of millions of years of evolutionary history.
These bacteria appear to be more resourceful than bacteria with respiratory metabolisms that live outside of a host organism, which mostly use inorganic compounds. The respiratory gut bacteria Light and team identified specialize in various organic metabolites, which makes sense given the constant food supply.
Chemically complex
“There is so much organic matter in the gut that comes from the food we eat. It’s chemically complex, and you need more enzymes to accommodate it in that environment,” Light said. “We think this variety of genes enables gut bacteria to use a lot of different things that come their way.”
Some of the metabolites they use also have interesting implications for human health in the gut. People with type 2 diabetes, for example, have higher levels of an amino acid byproduct called imidazole propionate in their blood. Another metabolite, resveratrol, impacts several metabolic and immune system processes, and itaconate is produced by macrophages in response to infections.
Light hopes that more research like this will help us understand the function of different microbes in the gut, which can in turn be leveraged to improve health.
“I’m hoping our understanding of these different metabolisms and how they work will enable us to come up with strategies to intervene – either through the diet or pharmacologically – to modulate the flow of metabolites through these various pathways,” he said. “So, in whatever context, like type 2 diabetes or following an infection, we could control which metabolites are being produced to have a therapeutic benefit.”





No comments yet