Researchers have revealed, using a combination of biochemistry, structural biology and computational modeling, how a particular riboswitch regulates its own synthesis.
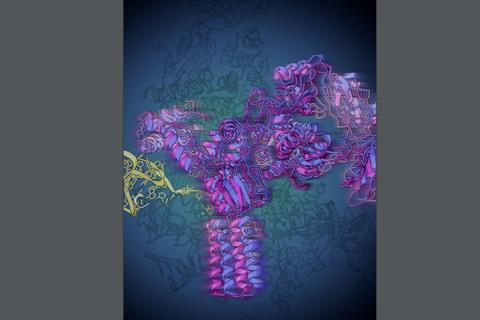
A promising target for new and improved antibiotics, riboswitches are small stretches of RNA that regulate a process necessary for the production of proteins by the bacterial cell.
Riboswitches are found almost exclusively in bacteria and could be targeted with antibiotics so that animals or humans are unaffected. With a full understanding of how riboswitches work, researchers may be able to develop drugs that disrupt the cellular machinery that creates needed proteins.
Regulating own synthesis
Now, researchers at the University of Michigan’s Department of Chemistry and the Life Sciences Institute have revealed, using a combination of biochemistry, structural biology and computational modeling, how a particular riboswitch regulates its own synthesis.
The first step in generating a protein from the genetic code is called transcription. The enzyme RNA polymerase (or RNAP) travels along the DNA, copying the genetic information found in DNA into a strand of RNA. During this process, RNAP will undergo several “pauses” as it waits for further instructions from the cell. Mechanisms for this pausing and restart have long remained elusive to scientists but promise to become a perfect target for antibiotics.
The team, led by chemistry professor Nils Walter through a collaboration with the labs of LSI professor Melanie Ohi and former U-M scientist Aaron Frank, used a structural biology technique called single particle cryo-electron microscopy (cryo-EM) to visualize for the first time how this transcriptional regulation occurs. Their results are published in Nature Structural & Molecular Biology.
Altering the shape
The Walter lab looked at a particular riboswitch that binds a molecule made by the cell, called preQ1. When the preQ1 molecule binds to the riboswitch it alters the shape of the RNA, which then allows the RNAP to once again continue along the DNA so that transcription continues.
Riboswitches were first discovered in 2002, but their specific roles related to the transcription machinery are not well understood. And it’s not hard to see why that is, says Adrien Chauvier, a Walter lab scientist and expert on riboswitches.
“This is a David vs. Goliath situation,” he said. “RNAP is this giant Goliath and the riboswitch is David. Because of this drastic size difference, visualizing where and how preQ1 regulates transcriptional pausing is equal to finding a needle in a haystack.”
Earlier research from the Walter lab revealed that transcriptional pausing is switched on and off as a function of the preQ1 molecule binding to the riboswitch. Moving forward, the Walter lab teamed up with cryo-EM expert Ohi to visualize what was happening.
Multidisciplinary collaboration
“This work is a great example of the strength of doing science at the University of Michigan. Three labs with different expertise were able to form a multidisciplinary collaboration that led to an important and novel discovery,” said Ohi, also a professor of cell and developmental biology at the U-M Medical School. “These findings wouldn’t have been possible without this synergy, along with the investments the university has put into strengthening cryo-EM and RNA biology at U-M in recent years.”
Single particle cryo-EM can determine the structures of large protein complexes by building 3D models from millions of 2D images of particles frozen in different orientations, revealing structures that contain molecular details that provide functional insights.
The structural information from single particle cryo-EM corroborated the Walter lab’s earlier findings, but also revealed a specific change in the shape of the riboswitch never seen before. When the preQ1 molecule binds, the riboswitch twists to communicate to the RNAP to continue transcription.
More precise understanding
These observations were further rationalized and validated through a collaborative effort with Frank, then a professor of biophysics and chemistry at the University of Michigan and an expert in computational modeling of RNAs. With detailed 3D models in hand, the U-M collaborative team now has a more precise understanding for how this riboswitch regulates transcriptional pausing.
“Now we understand the whole process of riboswitch regulation and can use that knowledge to specifically target these critical parts of bacterial life, hopefully averting the coming crisis of multidrug-resistant bacteria,” Walter said.
In addition to Walter and Chauvier, U-M researchers include Jason Porta, Indrajit Deb, Emily Ellinger and Katarina Mezei. This work was supported by the National Institutes of Health grants to Walter and Ohi, LSI and by the U-M Cryo-EM Biosciences Initiative.






No comments yet