In the relentless fight against microbial infections, our arsenal of antibiotics has expanded and contracted like the chest of a long-distance runner, oscillating between the use of broad and narrow-spectrum agents. This historical ambivalence, though underpinned by pragmatic intentions, has inadvertently fostered an environment conducive to bacterial resistance.
Moreover, it has precipitated a crisis—undermining the human microbiome and eliciting a disheartening withdrawal of pharmaceutical funding from antibiotic research in the 21st century. In stark contrast, the oncological field has embarked on a transformative journey evolving from non-selective, broadly toxic agents towards precision medicine, emphasizing minimal off-target effects. This apparent divergence in therapeutic evolution underscores a potential paradigm shift for antimicrobial therapy, suggesting a pressing need for a revolutionary reevaluation of our strategies in antibiotic development.
The evolving landscape of chemotherapy, marked by a decisive shift from indiscriminate cytotoxic agents to targeted therapies, echoes an unmistakable progression toward precision medicine. Early forays into chemotherapy mirrored the nascent stages of antibiotic development, employing broad-spectrum approaches like nitrogen mustards and antifolates that indiscriminately targeted rapidly dividing cells. However, unlike the antibiotic domain, oncology rapidly confronted the untenable consequences of this approach. Drugs designed to specifically target cancer cells, such as the BCR-ABL tyrosine kinase inhibitor Imatinib, have become the gold standard for treating chronic myeloid leukemia (CML), doing so with minimal impact on healthy cells (Druker et al., 2001). This pivotal shift, propelled by an acute recognition of the dire toxicities associated with non-specific treatments and a burgeoning understanding of cancer’s molecular underpinnings, ushered in a golden era of innovation in cancer treatment.
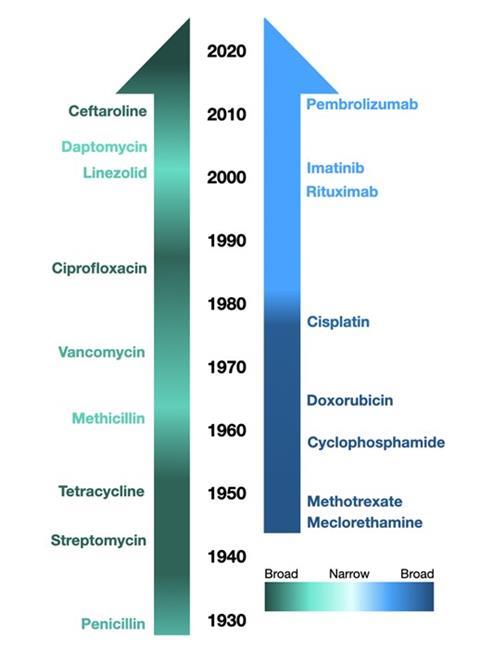
Conversely, the journey of antibiotic development has been marked by a persistent pendulum swing between broad and narrow-spectrum agents. The advent of Penicillin G in 1942 heralded an era of relatively narrow-spectrum antibiotics, exhibiting high selectivity for Gram-positive bacteria. The subsequent allure of broad-spectrum antibiotics, exemplified by Tetracycline, sought to extend our combative reach across a wider array of pathogens. Despite their utility, these agents significantly contributed to the escalating crisis of antibiotic resistance and disruptive impacts on the microbiome (Ventola, 2015). Broad-spectrum antibiotics, though versatile, indiscriminately devastate not only pathogenic bacteria but also the commensal microbiota that play crucial roles in our health, effectively blurring the line between cure and collateral damage (Belkaid & Hand, 2014). The clinical fallout from disturbed microbiomes, including heightened vulnerability to infections like Clostridioides difficile, underscores the unintended consequences of indiscriminate antibiotic use (Dethlefsen & Relman, 2011).
Both fields initially embraced broad-spectrum approaches but have since evolved divergently, reflecting a nuanced understanding of specificity, off-target effects, and the consequential impact on either the microbiome (in the case of antibiotics) or non-cancerous human cells (for chemotherapy). The antibiotic development timeline demonstrates a historical reverberation between broad and narrow-spectrum agents, whereas the evolution of chemotherapy illustrates a more harmonic and linear progression toward high specificity.
The analogy between cancer treatments and antibiotics extends beyond their therapeutic objectives; it encompasses our fundamental understanding of specificity and toxicity. In oncology, the conservation of healthy cells is paramount, a principle that has guided the development of targeted therapies. To align with this progression, the field of microbiology must adopt a perspective that views the human microbiome as a critical organ system, where disruption by non-targeted antibiotic activity equates to toxicity. Any inhibition of non-pathogenic bacteria by antibiotics should thus be viewed through a lens similar to the toxicity evaluations of chemotherapeutics towards non-cancer cells.
The narrative arc of antibiotic development, from the serendipitous discovery of Penicillin to the contemporary quandary of resistance, underscores a profound lesson: pathogens and cancer cells share more than a superficial resemblance in their defiance of therapeutic endeavors. They represent maladaptive elements within biological systems that demand precision in their rectification. Pathogens and cancer cells, in their essence, represent opportunistic entities within their respective ecosystems. Their survival strategies—ranging from resistance mechanisms in bacteria to the ability of cancer cells to evade apoptotic signals—emphasize their inherent capacity to adapt and flourish under adverse conditions. Just as cancer cells evade the immune system through mechanisms like antigenic variation and inducing immune tolerance, pathogenic bacteria deploy similar strategies—altering surface proteins and forming biofilms—to escape detection and eradication. This adaptability not only challenges our therapeutic interventions but also poses a significant risk to the host’s equilibrium.
Skeptics might argue that the inherent variability and adaptability of bacteria demand broad-spectrum antibiotics broad-spectrum antibiotics as a catch-all solution. However, this perspective overlooks the nuanced balance of microbial ecosystems and the long-term consequences of widespread antibiotic use. While the need for broad-spectrum coverage cannot be entirely dismissed, the continuous reliance on such agents without considering their broader impact has contributed to our current predicament. The oncology field’s emphasis on specificity, despite the heterogeneity and elusiveness of cancer, demonstrates that a focused approach does not preclude effectiveness (Vasan, Baselga, & Hyman, 2019). In fact, it enhances it by reducing the likelihood of resistance and off-target effects.
The imperative for antimicrobial development is clear: We need to recalibrate our approach to antibiotic development, championing the pursuit of specificity and minimizing off-target effects on the microbiome. Antimicrobial research requires a renaissance, a paradigm shift in how we conceptualize and evaluate antibiotic efficacy and toxicity, integrating lessons from our colleagues in oncology. By doing so, we can usher in a new era of antimicrobial therapy, one where victory in the battle against microbial infections does not merely denote eradication of pathogens but also preservation of the microbial allies that inhabit our bodies.
Further Reading:
The Antibiotic Resistance Crisis - PMC (nih.gov).
Role of the Microbiota in Immunity and Inflammation: Cell
A view on drug resistance in cancer | Nature
Conflict of Interest
Mathew Mitchell is a cofounder of Organicin Scientific, Inc. whose mission is to develop bacteriocins for use as alternatives to conventional antibiotics. He receives no salary for this position.






No comments yet