Undoubtedly, the gut microbiome is key to understanding the impact of diets and lifestyles on health. However, despite decades of scientific advancements, the connection between gut microbiome shifts and perceived health benefits remains unclear. Is the microbiome community inhabiting the gut just the sum of its parts? Would a healthy microbiome consist solely of beneficial microbes? Do fecal microbes represent those living in the gut?
This article highlights key points of misinterpretation and issues that require consensus for a better understanding of the gut microbiome’s impact on health.
For decades, microbiome research has focused on generally accepted approaches. Here, some of these assumptions are questioned. These building blocks of knowledge will need to be resolved to harness the full potential of the microbiome for human health and well-being.
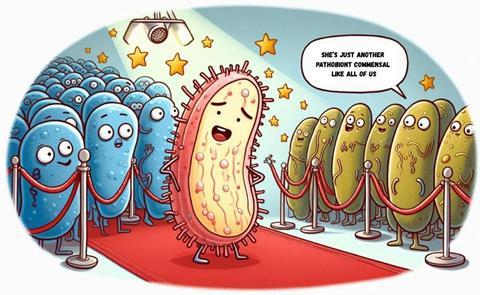
Friend or foe? Commensal or pathobiont?
The first concept to be challenged is the use of beneficial and harmful criteria to classify gut microorganisms. This has been a long discussion for decades. Scientists struggle to classify them. Sometimes, expressions like “pathobiont with commensal activity” are found in the literature.
There’s a general concept that increasing bifidobacteria or lactobacilli in your gut is the best way to balance your microbiome, since both are beneficial microorganisms. However, a beneficial bacterium does not always behave as expected. Bifidobacterium adolescentis may turn out to be harmful, acting as a pathobiont when the environment promotes disease.
Pathobionts, potentially harmful microorganisms, constantly interact with and challenge the immune system. When something disrupts this balance, inflammation prevails over tolerance. But if they were eradicated and never presented to the immune cells, tolerance would be lost, and the sole presence of these potential pathogens would cause inflammation and its deleterious consequences. In that order, a small burden of potentially harmful bacteria does seem to be required to keep our immune system trained. They must be part of the community. Otherwise, the immune system would become reactive to microorganisms that are not harmful when controlled. This loss of balance is being considered part of the pathogenesis of inflammatory bowel diseases.
Who are the pathobionts?
There’s also a discussion on the taxonomic level. One bacterial strain can be potentially pathogenic, while another one belonging to the same species is just a commensal. Let’s think about how to classify E. coli. It is the most well-known bacterial species harboring both commensal and enteropathogenic strains. We couldn’t say that one is harmful or beneficial without classifying them beyond the species level. In that sense, 16S sequencing is not an appropriate technique for counting commensals and pathobionts.
Fecal microbiome vs gut microbiome: what are we talking about?
There are two distinct samples representing the microorganisms inhabiting the gut. Microbial cells in our gut that mostly interact with immune cells are those attached to the mucosa, sometimes forming special structures called biofilms. Other microorganisms live in the gut lumen, in a free-floating mode. They remain in contact with the gut contents and are finally eliminated together with the stool. For decades, the scientific community has not distinguished between fecal microbiota and gut microbiota. Surprisingly, studies comparing both samples conclude that these communities differ: Durban et al 2010; Jiao et al 2022; Ingala et al 2018. Paljetak et al 2022 even found that compared with fecal samples, mucosal samples better resolved between patient groups.
The vast knowledge on the so-called “gut microbiome” comes from fecal samples. Little is known about the behavior of mucosal communities in health and disease. That knowledge comes mainly from cancer or IBS biopsies. To date, the scientific evidence suggests that fecal microbiota is shaped by diet, while mucosal microbiota drives host-gut microbiome interactions.
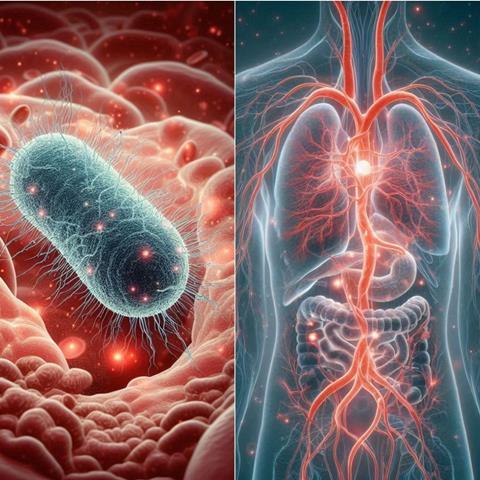
The gut microbiome axes: How do gut microbes connect with our body?
Gut microorganisms may directly interact with epithelial and immune cells in the mucosa, or indirectly by delivering more than 400 microbial metabolites that interact with local or distant tissues, entering the systemic circulation, and impacting distant organs. Most of them are amino acids, Short Chain Fatty Acids (SCFAs), bile acids, vitamins, and microbially modified metabolites. It remains unclear yet how these molecules are distributed, eliminated, and how they interact with cells of different tissues. Are they transformed into other molecules in the liver? Do these molecules have specific receptors, or are they a source of energy to different types of cells? Verbeke and col. have been actively contributing to this field, specifically exploring pre- and postprandial SCFA human serum levels, and their impact on disease diagnosis.
Conclusion
To better understand the gut microbiome and its health potential, there’s an urgent need for more sophisticated metagenomic tools that combine transcriptome, proteome, and metabolome technologies as well as bioinformatic tools to assess the microbiome as a functional community. Mucosal microbiome studies could be used to define more precise host enterotypes and to find signatures for disease diagnosis and monitoring, whereas fecal microbiome may better define dietary microbiome patterns and support dietary intake assessments. To further explore the intestinal microbiome, novel, less invasive methods must be developed, such as robot or intelligent capsules for monitoring the intestinal tract. Finally, studies on the physiology of serum microbial metabolites should be pursued to address the gut-organ axes in health and disease.
Conflict of interest: Dr. Gurovic is the scientific founder of Future Biome. The company develops mushroom-based functional ingredients that target the gut microbiome for human health.






No comments yet