The overarching goal of our research programme is to determine how locally derived complement proteins in the lung can be harnessed to ultimately reduce the burden of end-stage lung disease.
To this effect, a major focus of our work is on how epithelial cell-derived complement proteins in the lung modulate the severity of acute lung injury induced by different aetiologies, such as pneumonia. We are based in the Division of Pulmonary and Critical Care Medicine at Washington University School of Medicine but have active collaborations with investigators in the Divisions of Infectious Diseases, Gastroenterology and Rheumatology, and also in the Departments of Molecular Microbiology, Pathology and Immunology, and Surgery.

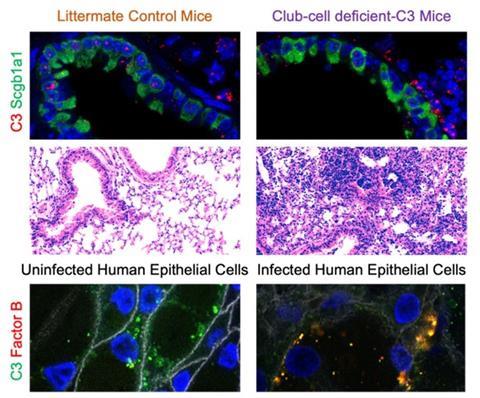
We use models of bacterial pneumonia as well as lung transplantation to investigate the effects of local complement production, activation and function. Using models of Klebsiella pneumoniae and Pseudomonas aeruginosa (Pa), we have demonstrated the protective effects of C3 and Factor B in bacterial pneumonia-induced acute lung injury. More recently, we reported that local complement production offers local protection in the lungs . Specifically, we used primarily human tracheobronchial epithelial cells, and mouse models of conditional gene deletion to investigate the contribution of local C3 production in the lung to protecting the pulmonary mucosal surface from cell damage caused by Pa infection. We reported that mice lacking C3 only in lung epithelial cells (specifically, club cells) exhibited more pronounced acute lung injury than control mice, even though circulating C3 levels were maintained at near-normal levels by maintenance of liver C3 production.
These findings build on our prior observations regarding the cytoprotective role of C3 in the lung and point to lung epithelial cell-intrinsic protective functions provided by C3 as an essential contributor to first-line defence against bacterial pneumonia. On further investigation, we demonstrated that the patterns of cellular injury and death occurring in C3 deficiency parallel what is seen in Factor B deficiency. We have demonstrated colocalisation of these proteins in airway epithelial cells during stress. How C3 and Factor B interact intracellularly to facilitate cell survival during infection is an active area of research in our laboratory.
At the same time, another arm of the laboratory focuses on the deleterious consequences of increased complement activation, as has been observed in lung transplantation and SARS-CoV-2 infection. In the context of SARS-CoV-2 infection, we have been investigating how mitochondrial DNA released from dying cells can activate complement, thereby perpetuating organ injury . Additionally, with Dr Andrew Gelman in the Division of Cardiothoracic Surgery, we are leveraging an orthotopic lung transplant model that develops obliterative bronchiolitis, to characterise how dysregulated complement activation may be driving chronic rejection.
In this context, our long-term goal is to identify the right targets and time to intervene in order to control this increased local complement activation and mitigate lung damage after transplantation. As a part of this work, we are also modelling how Pa infection post-lung transplantation breaks tolerance, to increase the risk of donor-specific antibodies and antibody-mediated rejection.
We utilise state-of-the-art technical modalities including live-cell imaging, flow cytometry, ELISA, protein- and DNA-based molecular biology, biochemistry, cell/tissue culture, fluorescence and confocal microscopy, and DNA/RNA sequencing to address these questions. Our division has longstanding experience in epithelial cell biology and cellular immunology, and we aim to integrate these divisional strengths with our expertise in complement biology and pneumonia. We look forward to collaborating with investigators from around the world, including sharing of reagents, in order to better understand the role of the complement system at mucosal surfaces, especially in but not restricted to the lung. For more details, please visit our website at https://sites.wustl.edu/kulkarnilab/.




No comments yet