Anthropologists, who study the human experience across time and space, are inclined to see life as a product of biological and cultural factors. We have argued that systems of human knowledge and practice have shaped forest vegetation, land composition, and atmospheric composition, even before our contemporary acceleration of carbon-fueled industry, capital accumulation, and biodiversity contraction.
Amid these sweeping studies of human conditions, it is also important to notice how humans shape life, and are shaped by life, on a much smaller scale. Microbes preceded us and evolved with us each step of the way. They’re part of our biological and cultural story too – none more so than those fermented foods that we carefully shape according to our particular tastes, local ingredients, and recipes. Asking about the significance of a pickle, kimchi, or yogurt as a biocultural landscape demands that researchers take seriously both the quantifiable diversity of microbial life as well as the diversity of stories, recipes, kitchens, and love poured into each bite.
Anthropology has a disciplinary bias toward holism. Yet even within anthropology as an academic field, qualitative sociocultural and quantitative biological researchers do not always combine their strengths to design research projects. This is a shame, because the interplay of cultural and biological forces give rise to many of our pressing 21st century challenges, from climate change to antimicrobial resistance. Neither approach can capture the full story in isolation.
Andrew Flachs, a cultural anthropologist, looks to food and agriculture systems to ask how culture shapes agricultural landscapes, through technologies and practices, through the improvisatory and repertory sets of knowledges that farmers employ given their political and economic circumstances. Joseph Orkin, a biological anthropologist, combines field and laboratory research to study issues of conservation and ecological function. Through his research into how populations of non-human primates shift amid changing climates and fracturing forests, he developed protocols to detect, collect, and study microbiome ecology in situ. While each of us had a longstanding interest in heritage, health, and food, it took us years of conversation to realise that we were often asking similar questions at different scales and with different tools. But just as human food practices or dietary context shape our larger ecology, we also create extreme salty and sour landscapes every time we make sauerkraut. What’s more, consuming that food shapes our own internal ecology. There is plenty of evidence to show that different diets and environmental contexts result in different human microbiomes, a finding with profound impacts for how we eat and what a healthy relationship to microbiological life looks like.
Like many great ideas, we sketched it out at the deli.
In taking fermentation seriously as a cultural and a microbiological process, we wanted to give equal weight to both the ecological compositions that we could track through quantitative microbiology and the stories of taste, variations in recipe, and personal meaning that created those foods in the first place. Importantly, we also knew that we wanted to study this process in real life as part of a regular diet – not in the controlled space of a clinical trial. If, as we suspected, minor differences in kitchens, recipes, or preparation resulted in different microbial ecologies, then we needed an approach capable of capturing this wide range in the variation of how people make and eat these foods.
While clinical trials can help us to precisely understand key variables that influence the form and function of the microbiome, we wanted to embrace the “noise” of variable palates, recipes, and work. People don’t live and eat outside of a clinical trial the same way they live and eat while enrolled in one. Such “confounds” in research linking food, culture, and microbiology are also the reality of everyday lived experiences. Still, we also did not want to tack too far into cultural narratives and lose sight of the factors that we could quantify. Our favorite kind of research embraces multiple perspectives to create a cohesive whole: insights into a heritage recipe or a journey of improvisational fermentation are made more meaningful when that story can be supported by a precise microbiological accounting. Similarly, long lists of species abundance and frequency can tell us a great deal about how a microbiome takes shape. But without cultural context we have no frame of reference for why and how these microbial communities are shaped, let alone how they come to produce culturally meaningful flavours and sentiments of home.
To begin answering both questions, we joined a 2018 workshop led by fermentation revivalist Sandor Katz. Andrew adapted methods from his prior work in sustainable food and agriculture systems: voice recorders for interviews, notebooks and a laptop to document reflections on participation and observation, a camera to capture a photographic record, and a mandolin to build non-academic relationships with other participants. Joe adapted methods from his work studying the microbiome among free-ranging nonhuman primates: liquid nitrogen to preserve a genetic snapshot of fermented foods, sterile gloves and pipettes to handle biological materials, and pre-filled ethanol tubes to capture the changing microbiome of our generously participating fermenters through daily stool samples.

Given the sensitive nature of the biological data we were collecting, we took care to preserve privacy in our data management. To keep personal identities and potential health data anonymous, we use pseudonyms in scientific articles. We additionally assigned color codes to each individual’s sample and dissociated identifiable personal information from the microbial analysis. This way, no genetic analyst, including Joe, has access to any identifiable information from workshop participants, and no qualitative data analyst, including Andrew, has access to the raw genetic data associated with human stools. We feel it is important to report this, as best practices will likely change in the future, and our intention and responsibility is always to the rights to privacy of our research interlocutors.
To quantify the diversity of life in our fermented foods, we collected 140 samples of fermented food and froze them in a vat of liquid nitrogen to capture a snapshot of the microbes swimming through kimchi or miso. Of course, to truly understand how these foods affect human bodies, we needed to know how much of this microbial diversity made its way into and through the digestive system. Each morning after coffee and breakfast, fermentation enthusiasts graciously donned gloves, jabbed sterilised chopsticks into sterile tip covers, and scooped a few milliliters of their morning stool into an ethanol-filled tube to preserve a picture of our changing microbial ecology. As participant-observers, the central method of cultural anthropology, we joined our research participants in the eating of fermented foods, carefully documented our own and others’ process in creating them, and collected our own stool samples.
Our research question itself was simple, even if the methods were complicated: how do stories and experiences with fermentation create different microbial possibilities?
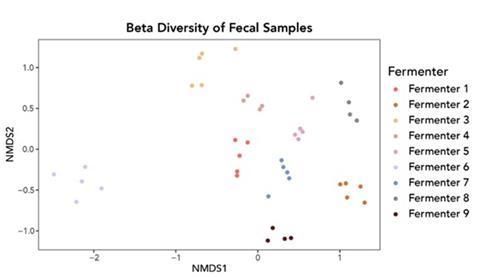
First, the microbial abundance and diversity found in the fecal and food samples indicate distinct communities. As extreme microbial landscapes, food samples were often dominated by a small number of taxa while the fecal microbial communities were more diverse. Fecal samples also clustered by individual, which suggests that while the broader gut microbial communities of the participants fluctuated throughout the workshop, homeostasis is powerful: diet alone was not a dominant determinant of composition over the few short days we spent talking, collecting, fermenting, and eating. In considering the experiences of fermenters, we benefit from an anthropological perspective on fermentation as a practice with socioecological consequences. Participants brought up notions of authenticity and place time and again in our discussions, and our approach offered a chance to link specific cultural values to microbiological consequences in practice.
“With the work that I do, it is kind of nice cooking. I mean, because even an act as simple as just slicing up cabbage can feel, like: well here’s what I’ve done,” said Matt[1], a pastor from Pennsylvania. “There’s just some immediate satisfaction when others can join you and then you can gather around a table. It’s really nice.” For others, fermentation provided an outlet for their creativity. “Initially I’ll look at a recipe and follow it, but I’m always riffing on it…what I’m feeling spontaneously in the moment.” During a later interview, another workshop-goer was more blunt: “I feel a little bit constitutionally unable to exactly follow a recipe.”
That creativity manifests in the non-homogenous tastes and practices that go along with these foods produced by the wide range of variation in bacterial genera that accompany different categories of fermented foods. Fermented food samples largely clustered according to their broader food category – fermented vegetables had one kind of profile while fermented grains had another. These clusters suggest that under real world conditions, different broad types of fermented foods provide similar bacterial ecosystems. However, the amount of time that a food fermented had a strong influence on microbial life and within these categories no two sauerkrauts were alike.
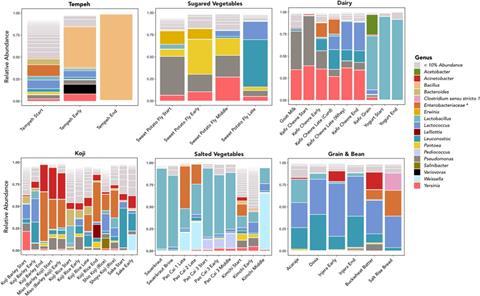
Interestingly, while different broad categories of foods had similar overall microbiological communities, like dairy, grain-based, or salted vegetable ferments, particular foods within these categories, like injera vs. dosa or kimchi vs. sauerkraut, varied in measurable ways, suggesting that small variations in food preparation may have a significant impact on the resulting microbiota even when these are prepared in the same kitchens. Through these small variations in preparation, home fermentation appears to foster a wide range of microbial landscapes where a variable set of microbes can thrive. Home ferments do indeed provide a mechanism for a more personalised, micro-biodiverse meal than that found in most commercial fermented foods, which are sterilised and then inoculated with specific bacterial strains to provide a uniformity of taste and consistency in regulatory approval. That is to say, not all fermented foods offer the same collection of biota or even the same kinds of potentially probiotic benefits in situ. That failure to control these environments might look like a failure to an experimental trial. For us, these variations and minor changes were precisely the phenomena that our design hoped to capture.
Throughout the fermentation process we observed substantial changes in microbial communities that coincided with increased fermentation time. In some cases, there was a stark transition from a highly diverse to a nearly uniform community. After 24 hours, once the tempeh had become completely covered in white mold, the bacterial community was almost entirely composed of the genus Bacillus. In similar fashion, the early-stage kimchi microbial community reflected a high number of low-abundance genera. But after a full day of fermentation, it came to be dominated by lactic acid bacteria, predominantly Weissella and Lactobacillus. We also observed some evidence that subtle changes in preparation can bring about major differences in microbial communities. Three different pao cai samples, mixtures of vegetables with Sichuan spices, salt and sugar, that had been fermenting for one week and six months had similar bacterial genera that differed in relative abundances; this could reflect differences in age or the seasonings used. In contrast, a pao cai, made during the workshop lacked the high abundance of Enterobacteriaceae found in the other samples and had a strong presence of an additional lactic acid bacteria, Pediococcus. Each of the three pao cai were eaten by workshop participants and differed slightly in flavor and texture. These represent both microbial differences and metabolomic consequences, all shaped by a cultural context. By combining microbiological and participatory approaches, we found that we could turn a confound (differences in preparation) into a research finding (differently desired outcomes).
The probiotic microbes we detected would likely be welcome and an affirming news to participants who stressed potential health benefits in consuming home-fermented foods. Further, our comparison of foods and stools suggests that, as in more controlled studies of the gut microbiome, these bacteria impact gut ecology over, at least, short periods of time. Our small, short-term, field study is not capable of determining if a gut microbiome could fundamentally shift as a result of regularly eating fermented foods. Each participant clearly had their own homeostasis and moved within a distinct personal range throughout the course of the study. Still, given the differences in gut microbiome observed between populations of humans in other research and the 25 genera we identified as having likely crossed between foods and guts, it is reasonable that fermented foods consumed in situ as part of a regular diet would influence gut microbiome composition.
The idea that landscapes are culturally formed is familiar to anthropologists – from this perspective, a petri dish is simply one more anthropogenic environment. We have simply focused on these interactions at a different scale. As conceived by a community of enthusiastic fermentation revivalists during a 2018 workshop, fermentation offers a space to embrace creative tactile work, consider one’s own body as a site of microbial transformation, and build relationships to both food production and microbial communities seen as beneficial. Variations within similar foods prepared at the same site as well as variations observed between different kinds of food suggest that this creative work, whether part of a traditional heritage practice or a newly discovered craft, results in a wide range of microbial communities within and without. Fundamentally, these cultural values matter for the kinds of microbiological communities we observed. In documenting all aspects of this process, we gain a fuller picture of how these ecologies are created and, importantly, why. As more kinds of teams collaborate, we hope that these multidisciplinary research designs continue to help researchers explore new dimensions of the world around and within us.
[1] Participants are pseudonymously named here






No comments yet