There’s something strangely soothing about watching urine flow through a biofilm-encrusted catheter. The soft ‘plip’ as the urine drips into the glass bladder, the low-level whir of the urine pump, and the gurgle of the water bath. Maybe if my PhD doesn’t work out I could pitch it as an alternative to whale music.
Visitors to our lab space at the University of Bath will often be greeted by the sight and smell of this catheterised bladder model system. A catheter is a flexible tube inserted through the urethra into the bottom of the bladder, then connected to a urine collection bag outside the body. They are essential medical devices used widely in hospitals, with as many as 25% of patients having a catheter inserted at some point during admission. Many community-based patients also rely on long-term catheterisation due to various chronic medical conditions that make natural bladder-emptying difficult or impossible.
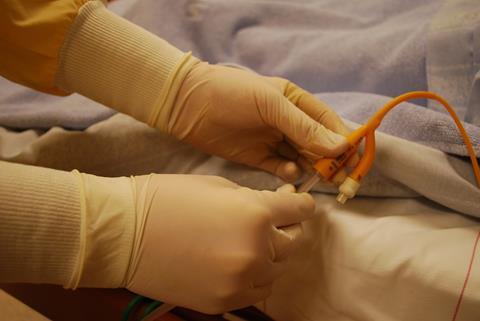
The presence of a catheter increases the likelihood that a urinary tract infection will develop, and catheter-associated urinary tract infections (CAUTIs) are the focus of our research group. A CAUTI occurs when the catheter becomes contaminated with bacteria from the gastrointestinal tract, allowing them direct access to the bladder and a constant supply of nutrients in the urine. Biofilm formation on the catheter surface improves survival and proliferation of the bacterial community due to avoidance of host defence mechanisms, and reduced susceptibility to antimicrobials. In addition, the risk of kidney infection is increased, as is the risk of developing life-threatening bloodstream infections and sepsis.
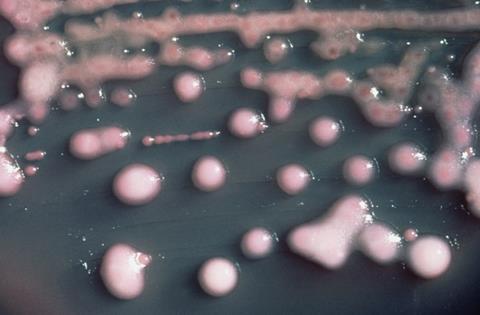
We are using several different angles to look at CAUTIs. Much of our research looks at Proteus mirabilis, the cause of 45% of CAUTIs and the most common cause of catheter blockage. If an infection caused by a slimy, gunked-up catheter tube wasn’t bad enough, the potent urease activity of P. mirabilis enables it to form a crystalline biofilm that can block the catheter completely. Urease raises urinary pH through the breakdown of urea to ammonia, resulting in the precipitation of substances within the urine. These are incorporated into the biofilm to form a mineralised matrix, and the resulting encrustation of the catheter can eventually block urine drainage. This leads to urinary retention and reflux of contaminated urine to the upper urinary tract, further increasing the risk of severe complications in an already vulnerable patient population.
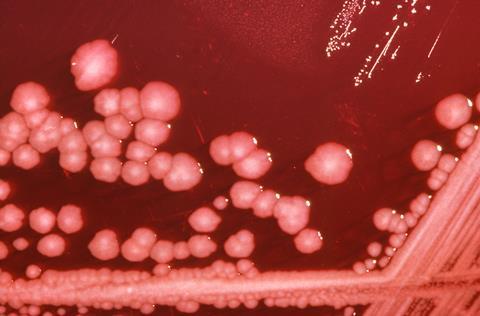
This crystalline biofilm formation means that P. mirabilis is an ideal species for use in the CAUTI bladder model system, as we can measure time to catheter blockage to compare treatments and interventions. Research supported by the Dunhill Medical Trust is investigating how catheter coatings could be used to reduce biofilm formation and blockage. The coating includes a coloured dye that is released when urinary pH rises, and would indicate to patients and healthcare staff that the catheter is likely to block. Ongoing work is looking at how antibiotics could also be included in these coatings.
We’re also looking to understand more about the characteristics of P. mirabilis that make it so good at forming biofilms on the catheter surface, particularly focusing on the role of efflux in biofilm formation. Efflux pumps are present in the cell membrane and export toxins and waste products from the bacteria. They are well known for their role in antimicrobial resistance, as by exporting antibiotics to the outside of the cell, the concentration inside the cell will decrease, along with treatment efficacy. This efflux process is also thought to be involved in biofilm formation, as the bacterial community is encompassed by a protective matrix of substances, which must be excreted from the cells within the biofilm. As these efflux pumps are present across different biofilm-forming pathogens, this work may also provide insight for a broader range of infections. The development of drug treatments that inhibit these efflux pumps could therefore be useful both in reducing biofilm formation, and in reducing resistance to antibiotics currently used to treat CAUTIs.
One of the current clinical strategies for reducing CAUTI incidence is the use of biocides such as chlorhexidine to flush through the catheter. This aims to kill bacteria present on the surface and reduce biofilm growth. But many P. mirabilis isolates show high tolerance to biocides, with catheter blockage able to occur despite chlorhexidine treatment. Another aspect of our research is investigating the mechanisms behind this tolerance, using clinical strains isolated from CAUTI patients. Changes to both lipopolysaccharide structure and efflux expression have so far been implicated, with ongoing work looking at how these lab findings translate to resistance against actual clinical products.
In a departure from the predominantly P. mirabilis focus of our group, we also have some work looking at biocide tolerance in a mixed-species community. This focuses on Klebsiella pneumoniae, a World Health Organization priority pathogen and common cause of CAUTIs. This is a new approach to investigate tolerance in the context of a polymicrobial infection, as this is also a clinically relevant scenario.
Another student in the lab said to me whilst we were discussing this article ‘I’m not sure I can give you a direct quote as they won’t appreciate the many expletives I use to describe my bladder model experience’. And it is very much the case that coming to the lab at 4 am to empty artificial urine out of a catheter drainage bag can make you question your life choices. But when so much of our work involves looking at obscure protein systems and metabolic pathways that most people have never heard of, I personally appreciate the reminder of its relevance to the bigger picture, and how our work really does have the potential to improve CAUTI treatments and lives in the future.










No comments yet