The COVID-19 pandemic caused by SARS-CoV-2 infection is marked by excessive inflammation of several organs, leading to multi-organ failure. The recognition of the virus by our bodies triggers severe immune responses, causing a ‘cytokine storm’, which can lead to acute lung injury and acute respiratory distress syndrome.
Conventionally, glucocorticoids (GCs) are used to reduce cytokine storms caused by COVID-19. But GCs also suppress the antiviral immune responses of the body, delay viral clearance from the lungs, and worsen viral pneumonia in patients. Therefore, an anti-inflammatory therapy that does not suppress antiviral immune response is necessary.
READ MORE: BNT162b2 vaccine not only targets COVID-19 virus, but may also help control innate inflammation
READ MORE: Study finds immune protein modification blocks viral replication, heart inflammation
To address this gap, a research team led by Professor Shinsuke Yasuda and Associate Professor Tadashi Hosoya, along with graduate student Seiya Oba, from the Department of Rheumatology, Graduate School of Medical and Dental Sciences at Institute of Science Tokyo (Science Tokyo), Japan, in collaboration with the National Institute of Infectious Diseases, explored the use of an anti-rheumatic drug—iguratimod (IGU)—in improving the survival of mice infected with SARS-CoV-2.
Risk factor
Obesity is one of the strongest risk factors for severe COVID-19. The research team has reported that visceral fat acts as a repository of inflammatory cells, making obesity a significant risk factor for COVID-19 in a previous study. Obese mice infected with SARS-CoV-2 exhibited a heightened risk of cytokine storm. Therefore, they decided to use SARS-CoV-2-infected obese mice as a model for cytokine storm in their current study. Their research was made available online on March 25, 2025, in the European Journal of Pharmacology and published on June 5, 2025, in Volume 996 on June 5, 2025
Eight-week-old male B6 mice were fed a high fat-diet (HFD) for ten weeks, following which they were injected with a sub-lethal dose of SARS-CoV-2. Drug treatments were administered to the mice 1 hour before virus inoculation up to 4 days after infection. Three days after infection, lungs were analyzed, or mice were monitored up to 10 days for recording survival rates.
“We evaluated the efficacy of dexamethasone (DEX)—a clinically used anti-rheumatic drug, IGU, a proinflammatory response inhibitor, H-151, and a control, respectively, after administering them to HFD-B6 mice,” Yasuda explains the experimental treatment groups of their study.
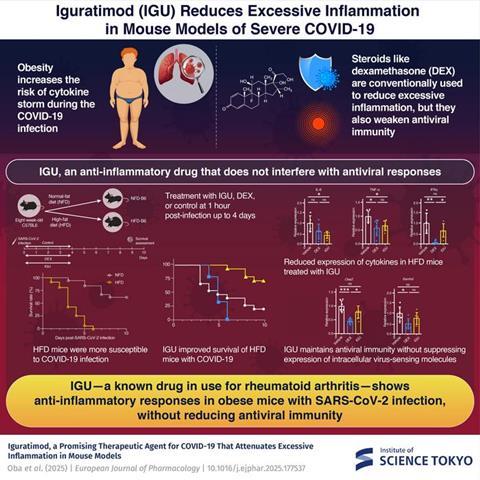
Mice treated with IGU showed higher survival rates than other treatment groups and demonstrated relatively mild inflammation and lung damage compared to the other groups. Hosoya further elaborates on their results, “We performed immunohistochemical staining of SARS-CoV-2 N antigen in the lungs 3 days after infection to understand whether IGU could suppress viral expansion in the lungs and found that the ratio of SARS-CoV-2 antigen-positive cells significantly decreased in the IGU group.”
Inflammatory responses
The research team also showed that in mice administered with IGU, infection-induced genes that support antiviral immunity were not suppressed, unlike in those treated with DEX. They observed that DEX suppressed inflammatory responses in SARS-CoV-2-infected mice while simultaneously weakening antiviral immune responses, leading to reduced survival rates and poor prognosis in this group.
IGU is a safe drug already approved for use in treating rheumatoid arthritis. This study points towards its efficacy as an immunomodulatory drug for patients with severe COVID-19-related cytokine storm. With further clinical studies, IGU may be repositioned as a promising therapeutic agent for SARS-CoV-2 infections.







No comments yet