Acoustic waves may be able to control how particles sort themselves. While researchers have been able to separate particles based on their shape — for example, bacteria from other cells — for years, the ability to control their movement has remained a largely unsolved problem, until now.
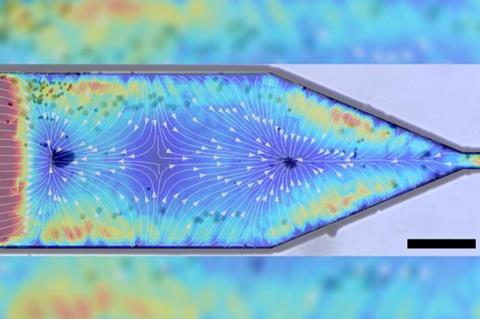
Using ultrasound technology and a nozzle, Penn State researchers have separated, controlled and ejected different particles based on their shape and various properties. They published their results in the journal Small.
“We engineered a microchannel nozzle and applied ultrasound energy to the system,” said corresponding author Igor Aronson, the Penn State Dorothy Foehr Huck and J. Lloyd Huck Chair Professor of Biomedical Engineering and professor of chemistry and of mathematics. “The nozzle plays two roles. It concentrates fluid flow, which is something other researchers have done. But in addition to that, the walls of the nozzle reflect the acoustic waves of the ultrasound energy.”
Nanorod model
Aronson and his collaborators worked with tiny materials called nanorods, which are some of the most studied synthetic self-propelled particles, according to Aronson. Because they are a similar size and have a similar swimming speed to bacteria, Aronson said, many of the conclusions drawn from observing nanorods can be applied to bacteria movement. For this reason, they are often used as proof of concept for future separation tasks.
In this case, the nanorods were half platinum and half gold. The researchers placed the nanorods in a nozzle, shaped like a miniature syringe, and then added hydrogen peroxide. The hydrogen peroxide is decomposed — or burned — on the platinum half of each nanorod, forcing them to swim in an imitation of how bacteria behave.
The researchers applied ultrasound to the nozzle, producing acoustic waves that, along with the flow of fluid, were able to separate the nanorod particles, aggregate them or extrude them from the nozzle.
Behaving differently
“The separation concept relies on the fact that nanorods and spherical particles have different responses to acoustic radiation and generated fluid flow,” Aronson said. “By controlling the nozzle shape and the frequency and amplitude of the acoustic radiation, we can coerce particles of different shapes and material properties to behave differently. This, especially, applies to active particles such as nanorods: They can swim autonomously, and their control is especially challenging.”
This level of control in separating out particles had not been demonstrated previously, according to the researchers.
Aronson said this demonstration has implications for future technologies, including additive manufacturing, also known as 3D printing, and drug delivery.
“For 3D printing, the idea is you can add certain additives to the ink — for example, nanorods,” he said. “So now, we could separate nanorods from spherical particles to deposit only some in the printout, such as depositing polymers without nanorods and so on, all to change the property of the printout.”
Bioprinting implications
Aronson said this principle also applies to printing living cells, known as bioprinting.
“Potential bioprinting applications may include designing acoustic nozzles for bio-inkjet-like printers,” he said. “By controlling the acoustic radiation in the nozzle, we can potentially extrude certain types of cells — for example, stem cells — and trap other types — for example, bacteria. It’s an additional control for bioprints.”
This capability could also be useful for separating bacteria from cells in targeted drug delivery, Aronson said. The researchers next plan to mix live bacteria and cells in a lab setting and then separate and control them.
The paper’s other authors are Leonardo Dominguez Rubio, a graduate student in the Penn State Department of Biomedical Engineering; Ayusman Sen, the Verne M. Willaman Professor of Chemistry at Penn State; and Matthew Collins, who was a Penn State chemistry graduate student at the time of this work. The U.S. Department of Energy supported this work.





No comments yet