The work not only reveals principles of evolutionary biology but also suggests a new strategy to combat the antibiotic resistance crisis, which kills an estimated 1.3 million people per year worldwide.
Findings, supported in part by federal funding, are published Nov. 20 in Science.
Members of the labs of Michael Baym, associate professor of biomedical informatics, and Johan Paulsson, professor of systems biology, devised a way to track the evolution and spread of antibiotic resistance in individual bacteria by measuring competition among plasmids.
Driving evolution
Plasmids are self-replicating genetic elements that float separately from a bacterium’s own chromosomes. Plasmids evolve independently but also help drive bacterial evolution, including the development of resistance to antimicrobial compounds. In fact, they are the primary way that resistance can jump from one type of bacteria to another.
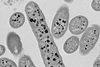
Scientists have suspected that competition among plasmids within bacterial cells is key to propelling plasmid evolution, but until now they hadn’t found a way to study it. First author Fernando Rossine, research fellow in biomedical informatics in the Baym Lab, and colleagues did so by solving two challenges.
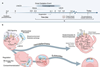
First, they created starting conditions in which each bacterial cell contained equal proportions of two plasmids that would compete with each other. Second, they used microfluidic devices to isolate single cells and better distinguish the effects of the intracellular plasmid competition.
Properties and constraints
The system allowed the team to discover basic properties of — and constraints on — plasmid and bacteria fitness and evolution. These constraints could inform new strategies that interfere with plasmid evolution and thus curb plasmids’ ability to learn to withstand antibiotics — potentially leading to treatments for life-threatening bacterial infections.
“The study provides us with new tools to fight and prevent antibiotic resistance by weaponizing the intracellular competition between mobile genetic elements themselves,” Rossine said.
From a more philosophical perspective, he added, the study illuminates how evolution operates at multiple, sometimes conflicting, levels, “which is fundamental for our understanding of complex life.”
Baym is senior author of the study. Additional authors are Carlos Sanchez and Daniel Eaton, who contributed to the work as Harvard Kenneth C. Griffin Graduate School of Arts and Sciences PhD students in the Paulsson Lab through the Systems, Synthetic, and Quantitative Biology program at HMS.
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health (grant R35GM133700), David and Lucile Packard Foundation, Pew Charitable Trusts, Alfred P. Sloan Foundation, and National Science Foundation (grant MCB-721 2426105).






No comments yet