Proteins sourced from microorganisms are attracting attention for their potential in biomanufacturing a variety of products, including pharmaceuticals, industrial enzymes, and diagnostic antibodies. These proteins can also be used for converting resources into biofuels and bioplastics, which could serve as viable alternatives to petroleum-based fuels and products.
Therefore, efficiently producing microbial proteins could make a significant contribution to sustainable manufacturing.
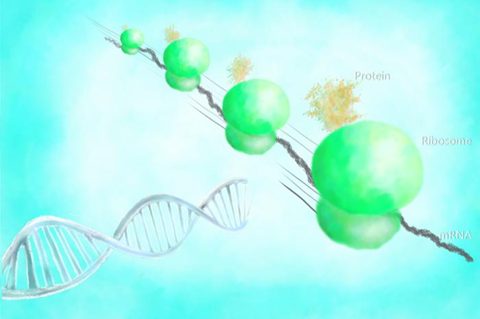
Producing proteins from Escherichia coli (E. coli) has become popular due to its cost-effectiveness and efficiency. However, yields of protein production in E. coli may be reduced depending on the specific gene sequence of the target protein.
In a study published in the journal RSC Chemical Biology, a research group in Japan has successfully developed a new technology that improves the efficiency of protein production in E. coli.
Ribosome stalling
The reduction in protein production is believed to be a contributing factor to ribosome stalling. Ribosomes synthesize proteins using genetic information carried by messenger RNA (mRNA). However, when ribosomes are unable to continue the translation process for some reason, protein synthesis is halted.
“In our previous research, we found that adding a short peptide sequence composed of four amino acids—serine, lysine, isoleucine, and lysine—to the N-terminus of a protein reduces ribosomal stalling and significantly improves translation efficiency in E. coli,” said Associate Professor Teruyo Ojima-Kato from Nagoya University’s Graduate School of Bioagricultural Sciences.
Based on this finding, Kato and Professor Hideo Nakano of Nagoya University, in collaboration with researchers from the National Institute of Advanced Industrial Science and Technology and Waseda University, conducted a study to identify short translational-enhancing peptides (TEPs) that can prevent ribosome stalling.
Tetrapeptide library
The researchers first created a tetrapeptide library, a collection of random peptide sequences formed by all possible combinations of the four amino acids. This library contains the arrangements of the 20 amino acids that make up proteins, totaling 160,000 distinct tetrapeptides. Using this library, they conducted a comprehensive analysis and identified several novel TEPs that effectively prevent ribosome stalling.
Next, the researchers attempted to evaluate the translation-enhancing effects of the 160,000 tetrapeptides. To assess such large numbers of tetrapeptides, they developed an artificial intelligence (AI) prediction model based on data from about 250 experiments.
The research group conducted three rounds of AI predictions, demonstrating that their AI model accurately predicts the strength of translation enhancement for all 160,000 tetrapeptides. This result suggests that AI-based predictive models could be helpful in the rational design of peptide sequences that can be readily translated into target proteins.
Sustainable manufacturing
“We present a novel approach for efficient production of proteins using short peptide sequences,” said Kato. “This technique can be applied to more efficient production of enzymes, which play a significant role in the biorefinery sector that generates chemicals and fuels from renewable resources. Our findings could provide fundamental technology to support sustainable manufacturing that does not depend on petroleum.”
Topics
- Artificial Intelligence & Machine Learning
- Asia & Oceania
- Bacteria
- Climate Action
- Economic Equality
- Escherichia coli
- Hideo Nakano
- Industrial Microbiology
- Nagoya University
- National Institute of Advanced Industrial Science and Technology
- Research News
- ribosome stalling
- short peptide sequences
- Teruyo Ojima-Kato
- translational-enhancing peptides
- Waseda University





No comments yet