Bacteria of the genus Streptomyces produce chemical substances called arginoketides, to which many other microorganisms react - bacteria form biofilms, algae join together to form aggregates, and fungi produce signalling substances that they would not otherwise produce, triggering new responses from other organisms.
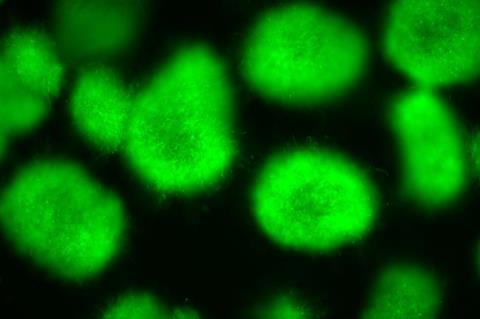
Researchers at the Leibniz Institute for Natural Product Research and Infection Biology (Leibniz-HKI) show this in a study, in which they investigated various Streptomyces species, the arginoketides they produce and their influence on other soil microorganisms.
Even though microorganisms cannot speak, they communicate with each other. To do so, they use chemical substances that other microorganisms understand as signals.
“These are relatively small organic compounds, known as natural products,” explains Axel Brakhage, head of the study and director of the Leibniz-HKI as well as a professor at the Friedrich Schiller University Jena. “Microorganisms produce a variety of such compounds and we are just beginning to understand this language.”
Super-communicators
Bacteria of the genus Streptomyces are apparently particularly important for communication in the soil. They are found all over the world and produce many different arginoketides, as was recently discovered by researchers at the Leibniz-HKI in a study published in Nature Microbiology.
Arginoketides are a subgroup of polyketides, a group of natural products produced by various organisms. Many polyketides are of medical interest because they, for example, are antibiotic or act against cancer cells.
The group of arginoketides identified by the Leibniz-HKI team triggers various processes in the soil.
“In previous studies, we have already seen that the fungus Aspergillus nidulans produces some substances only in the presence of streptomycetes,” says Maria Stroe, one of the two lead authors of the study. The arginoketide azalomycin F was identified as responsible for this.
Modes of signalling
For the current study, the researchers therefore investigated whether streptomycetes produce other compounds that are active as signalling substances.
“Through a literature search, we found a large number of examples where Streptomyces species worldwide produce structurally similar compounds or at least have biosynthetic gene clusters for corresponding arginoketides,” explains Mario Krespach, lead author of the study.
The researchers isolated some of these compounds from Streptomyces strains from soil samples and successfully tested them on the mould Aspergillus nidulans - they also triggered the production of chemical substances in the fungus that it does not otherwise produce.
“We therefore suspected that we may have found a general mechanism of microbial communication,” says Lukas Zehner, another author of the study.
Fungi respond
And indeed, the team found a large number of fungi in soil samples that formed substances in the presence of Streptomyces iranensis that they do not form otherwise. If the researchers switched off the corresponding biosynthesis genes for arginoketides, the effect did not occur.
Previous studies showed numerous activities of arginoketides - for example, they cause a fungus and a green alga to enter into a symbiosis, another fungus changes its shape and a bacterium forms a biofilm in response to the substances.
“We are now trying to understand the effects on the composition of microbial communities, the microbiomes, both from arginoketide production itself, and also from the substances produced from fungi in response,” says study leader Brakhage.
Implications for multicellularity
For example, one of the substances produced by Aspergillus nidulans inhibits a plant-pathogenic fungus. The effects of arginoketides on algae and fungi may also have contributed to the evolution of lichens and multicellularity.
“Elucidating this interplay helps us understand, among other things, how microbial communities are structured and how they help prevent plant diseases. In addition, we discover entirely new substances when we study the coexistence of microorganisms instead of just looking at isolated organisms,” Brakhage explains.
The study was supported by the German Research Foundation as part of the Balance of the Microverse Cluster of Excellence and the Collaborative Research Centres FungiNet (Transregio) and ChemBioSys, as well as by the Leibniz Association as part of the Leibniz Competition.




No comments yet